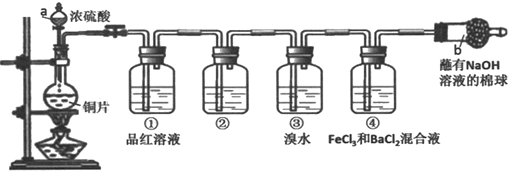
��Ŀ����
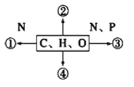
����Ŀ��������Ԫ��W��X��Y��Z��ԭ�������������ӣ�����ЩԪ����ɵij������ʵ�ת����ϵ����ͼ,����a��b��d��gΪ�����aΪ����ɫ���壬c��Z�ĵ��ʣ������ȷ�Ӧ�г�����������e��fΪ�������嵥�ʡ������й�˵����ȷ����

A. �����ӵİ뾶��Y>Z>X
B. ���⻯��ķе㣺Y>X
C. ����������Ӧˮ����ļ��ԣ�Z>Y
D. W��Y��������������ѧ��������ͬ
���𰸡�B
��������������������Ԫ��W��X��Y��Z��ԭ�������������ӣ�����a��b��d��gΪ�����aΪ����ɫ���壬��aΪ�������ƣ�c��Z�ĵ��ʣ������ȷ�Ӧ�г�����������cΪ����þ��e��fΪ�������嵥�ʣ����ݿ�ͼ��þ�뻯����b��Ӧ�������嵥��f����fΪ���������������뻯����b��Ӧ�������嵥��e����eΪ��������bΪˮ��dΪ�������ƣ�gΪ������þ��
��⣺��������������aΪ�������ƣ�bΪˮ��cΪþ��dΪ�������ƣ�eΪ������fΪ������gΪ������þ����WΪH��XΪO��YΪNa��ZΪMg��A. XΪO��YΪNa��ZΪMg�������Ӿ�����ͬ�ĵ��Ӳ�ṹ�����Ӱ뾶��X>Y>Z����A����B. �⻯�������ӻ�����е����ˮ����B��ȷ��C.Ԫ�صĽ�����Խǿ������������Ӧˮ����ļ���Խǿ�����ԣ�Y>Z����C����D.ˮ��������ⶼ�ǹ��ۻ����ֻ���й��ۼ��������ƻ�������ƶ��������ӻ�����������Ӽ�����ѧ�����Ͳ�ͬ����D����ѡB��

 ������������Ծ�ϵ�д�
������������Ծ�ϵ�д� �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д�����Ŀ�������������Ǵ�����Ⱦ��֮һ���û���̿��һ����̼��ԭ��������ɷ�ֹ������Ⱦ���ش��������⣺
��֪��2C(s)+O2(g)=2CO(g) ��H=- 221 kJ/mol
C(s)+O2(g)=CO2(g) ��H=- 393.5 kJ/mol
N2(g)+O2(g)=2NO(g) ��H= +181 kJ/mol
��1����ij��Ӧ��ƽ�ⳣ������ʽΪK=![]() ����д���˷�Ӧ���Ȼ�ѧ����ʽ��___________________________�����д�ʩ�ܹ�����˷�Ӧ��NO��ת���ʵ���(����ĸ����)_________��
����д���˷�Ӧ���Ȼ�ѧ����ʽ��___________________________�����д�ʩ�ܹ�����˷�Ӧ��NO��ת���ʵ���(����ĸ����)_________��
a.��������ѹǿ b.�����¶� c.ʹ�����ʴ��� d.����CO��Ũ��
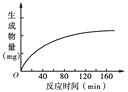
��2�����ݻ�Ϊ2L���ܱ������м������̿(����)��NO��������ӦC(S)+2NO(g)![]() N2(g)+CO2(g)��NO��N2�����ʵ����仯���±���ʾ��
N2(g)+CO2(g)��NO��N2�����ʵ����仯���±���ʾ��
���ʵ���/mol | T1/�� | T2/�� | |||||
0 | 5 min | 10 min | 15 min | 20 min | 15 min | 30 min | |
NO | 2.0 | 1.16 | 0.80 | 0.80 | 0.50 | 0.40 | 0.40 |
N2 | 0 | 0.42 | 0.60 | 0.60 | 0.75 | 0.80 | 0.80 |
��0��5min�ڣ���CO2��ʾ�ĸ÷�Ӧ����v(CO2)=______���������µ�ƽ�ⳣ��K=_______________��
�ڵ�15 min���¶ȵ�����T2�����ݱ仯���ϱ���ʾ����T1_______ T2(�>������<����=��)��
����30minʱ������T2���䣬����������ټ�������ַ�Ӧ������2 mol�����ʱ��Ӧ_______�ƶ�(�������������)�����մ�ƽ��ʱNO��ת����a=______________��
��3����ҵ�Ͽ����ð�ˮ��ȥ��ӦC(s)+2NO(g)![]() N2(g)+CO2(g)�в�����CO2���õ�NH4HCO3��Һ����ӦNH4++HCO3-+H2O
N2(g)+CO2(g)�в�����CO2���õ�NH4HCO3��Һ����ӦNH4++HCO3-+H2O![]() NH3��H2O+H2CO3��ƽ�ⳣ��K=________________��(��֪������NH3��H2O�ĵ���ƽ�ⳣ��Kb=2��10-5�� H2CO3�ĵ���ƽ�ⳣ��Ka1=4��10-7��Ka2=4��10-11)
NH3��H2O+H2CO3��ƽ�ⳣ��K=________________��(��֪������NH3��H2O�ĵ���ƽ�ⳣ��Kb=2��10-5�� H2CO3�ĵ���ƽ�ⳣ��Ka1=4��10-7��Ka2=4��10-11)