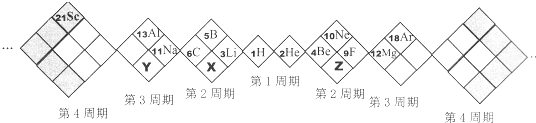
��Ŀ����
����Ŀ���ظ����(K2Cr2O7)��һ�ֳ�����ǿ��������������������������������ʡ�ij��ȤС��ģ����ҵ����������ˮ����Ҫ��Cr2O72-��Cr3+)��ͬʱ����ظ���ؾ�����������£�
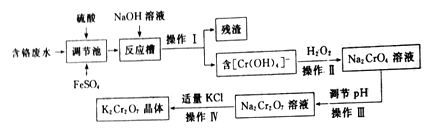
�ش��������⣺
��1������I��______����������Ҫ�ɷ���______��
��2�����ڳ��з�������Ҫ��Ӧ�����ӷ���ʽΪ__________________��
��3��������__________����ܡ����ܡ��������������ҺpH��ԭ����___________��
��4����Na2Cr2O7��Һ�л��K2Cr2O7����IJ��������Ǽ�������KCl���壬���衢�ܽ⣬��ˮԡ�ϼ���Ũ����________ʱֹͣ���ȡ����������K2Cr2O7������Ҫ��һϵ�в����У������������ܻ��õ�����__________�����ţ���
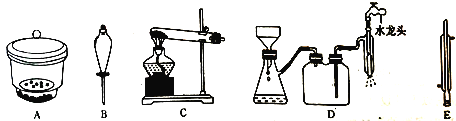
��5��Ϊ�������ˮ�Ƿ�ﵽ�ŷű���ijͬѧ����������ʵ�飺ȡ100mL������ķ�Һ��Ʒ����ƿ�У���Ũ�������pH=5�������������忹��Ѫ�ᣬʹCr2O72-��ȫת��ΪCr2+������cmol�� L-1��EDTA����H4Y��ʾ������Һ���еζ����䷴Ӧԭ��Ϊ��Cr3++Y4-=CrY-����ʵ������EDTA����ҺVmL��������ķ�Һ�к���Ԫ��Ũ��Ϊ______mg��L-1���ú�c��V��ʽ�ӱ�ʾ����
���𰸡� ���� Fe(OH)3 6Fe2++Cr2O72-+14H+=6Fe3++2Cr3++7H2O ���� ��������Һ��Cr2O72-����ǿ�����ԣ����������� ��Һ������־�Ĥ AD 520cV
�����������⿼�黯ѧ�������̣���1������I�õ���������Һ����˲���I�ǹ��ˣ��ظ����(K2Cr2O7)��һ�ֳ�����ǿ���������������������������ڳ���Cr2O72����Fe2��������Fe3������������ԭ��Cr3������Ӧ���м���NaOH��Һ��Fe3����OH����Ӧ����Fe(OH)3��������˲����ijɷ���Fe(OH)3����2������(1)�ķ��������ӷ�Ӧ����ΪCr2O72����Fe2����Fe3����Cr3�������ݻ��ϼ�������������ƽ����Cr2O72����6Fe2����6Fe3����2Cr3��������FeSO4��ͬʱ����������H2SO4��������ӷ���ʽΪ��6Fe2��+Cr2O72��+14H��=6Fe3��+2Cr3��+7H2O ����3���������(K2Cr2O7)��һ�ֳ�����ǿ���������������������������ᣬNa2CrO4��Һ����2CrO42����2H��![]() Cr2O72����H2O������ǿ�����ԣ��ܰ�������������˲������������pH����4��Na2Cr2O7�Ʊ�K2Cr2O7��˵��K2Cr2O7���ܽ��С��Na2Cr2O7����˵����ȳ��־�Ĥʱ��ֹͣ���ȣ��������IJ�������ȴ�����ˣ������Ҫ��������A��D����5������Ԫ���غ�ͷ�Ӧԭ����������ϵʽΪCr2O72����2Cr3����2Y4����2H4Y����˸�Ԫ�ص�����ΪV��10��3��c��52��103mg=52Vcmg����Ũ��Ϊ52Vc/(100��10��3)mg��L��1=520Vc mg��L��1��
Cr2O72����H2O������ǿ�����ԣ��ܰ�������������˲������������pH����4��Na2Cr2O7�Ʊ�K2Cr2O7��˵��K2Cr2O7���ܽ��С��Na2Cr2O7����˵����ȳ��־�Ĥʱ��ֹͣ���ȣ��������IJ�������ȴ�����ˣ������Ҫ��������A��D����5������Ԫ���غ�ͷ�Ӧԭ����������ϵʽΪCr2O72����2Cr3����2Y4����2H4Y����˸�Ԫ�ص�����ΪV��10��3��c��52��103mg=52Vcmg����Ũ��Ϊ52Vc/(100��10��3)mg��L��1=520Vc mg��L��1��

 ���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д�
���Ŀ��ּ�����ҵ�����ҵ����������ϵ�д� ����ѵ��ϵ�д�
����ѵ��ϵ�д� ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�����Ŀ��1��2-����������������������Ӽ�����ͼΪʵ�����Ʊ�1��2-���������װ�Dͼ�� ͼ�з�Һ�ƶ�����ƿa�зֱ�װ��ŨH2SO4����ˮ�Ҵ���dװ�D�Թ���װ��Һ�塣

��֪��CH3CH2OH![]() CH2=CH2��+H2O��2CH3CH2OH
CH2=CH2��+H2O��2CH3CH2OH![]() CH3CH2OCH2CH3+H2O
CH3CH2OCH2CH3+H2O
��������б����£�
�Ҵ� | 1��2-�������� | ���� | �� | |
״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� | ����ɫҺ�� |
�ܶ�/g��cm-3 | 0.79 | 2.18 | 0.71 | 3.10 |
�е�/�� | 78.5 | 131.4 | 34.6 | 58.8 |
�۵�/�� | -114.3 | 9.79 | - 116.2 | -7.2 |
ˮ���� | ���� | ���� | �� | ���� |
��1��ʵ����ӦѸ�ٽ��¶����l��170�����ҵ�ԭ����______________________________��
��2����ȫƿb��ʵ�����ж������á���һ���Լ��ʵ�������dװ�D�е����Ƿ���������
��д����������ʱƿb�е�����_______________________________�����ʵ��ʱdװ�D�е��ܶ���������Ϊ���ܵ�ԭ���Ǣ�_______________________________________________����ȫƿb�������������Ǣ�__________________��
��3������c��e�ж�ʢ��NaOH��Һ��c��NaOH��Һ��������________________________________��
��5����ȥ����������δ��Ӧ��Br2�����е���Ҫ����Ϊ___________��Ҫ��һ���ᴿ�����в����б������_____________ ������ĸ����
A���ؽᾧ B������ C����ȡ D������
��6��ʵ����Ҳ���Գ�ȥdװ�D��ʢ��ˮ���ձ�����Ϊ����ˮֱ�Ӽ��뵽dװ�D���Թ��У��� ��ʱ��ˮ����������ȴ1��2-��������������⣬��������������____________________________��