��Ŀ����
(8��)��������Ũ�Ⱦ�Ϊ0.5mol/L��������Һ����NaHCO3����C6H5ONa���� NH4HCO3���� NH3��H2O���� HCl
(1)����������Һ�м��ܸ����ᷴӦ���ܸ�NaOH��Һ��Ӧ���� ������ţ���
(2)�����ڵ�ˮ��Һ�ʼ��Ե�ԭ���ǣ������ӷ���ʽ��ʾ�� ��
(3)�۸�������NaOHŨ��Һ�ڼ��������·�Ӧ�����ӷ���ʽ�� ��
(4)ȡ10mL�ݵ���Һ��ˮϡ�͵�500mL�������Һ����ˮ�������c(H+)= mol/L.
(1)����������Һ�м��ܸ����ᷴӦ���ܸ�NaOH��Һ��Ӧ���� ������ţ���
(2)�����ڵ�ˮ��Һ�ʼ��Ե�ԭ���ǣ������ӷ���ʽ��ʾ�� ��
(3)�۸�������NaOHŨ��Һ�ڼ��������·�Ӧ�����ӷ���ʽ�� ��
(4)ȡ10mL�ݵ���Һ��ˮϡ�͵�500mL�������Һ����ˮ�������c(H+)= mol/L.
��1���٢ۣ�2�֣�
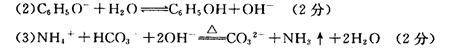
��4��10��12��2�֣�
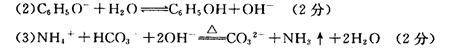
��4��10��12��2�֣�
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
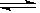 2H++S2��
2H++S2��