��Ŀ����
8��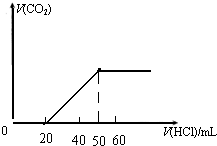 ��ʵ���ҳ��õ��������ܶ�1.20g•cm-3����������36.5%��
��ʵ���ҳ��õ��������ܶ�1.20g•cm-3����������36.5%����1�����������Ȼ��⣨��״����ͨ��1.00Lˮ�пɵõ�36.5%�����ᣨ����һλС������
��2��ȡ10mL36.5%�����ᣬ��ˮϡ����300mL����ϡ�ͺ���������ʵ���Ũ���Ƕ��٣�
����5.68g̼��ƺ�̼��þ��ɵĻ�����м���������I��ϡ�ͺ�����ᣬ��������CO2����ͨ��500mL ��NaOH��Һ�У�������ȫ�����գ���ȡ�˷�Ӧ�����Һ100mL���������Һ����μ���I��ϡ�ͺ�����ᣬ������CO2�������������������������������֮��Ĺ�ϵ��ͼ��ʾ��
��1��500mL NaOH��Һ�����ʵ���Ũ���Ƕ��٣�
��2��ԭ�������̼��Ƶ����������Ƕ��٣�
���� ��1������Ҫ�Ȼ�������ΪVL������n=$\frac{V}{Vm}$����HCl�����ʵ������ٸ���m=nM����HCl������������m=��V����ˮ������������������Һ���������ٸ������������з��̼��㣻����c=$\frac{1000��w}{M}$�������������ʵ���Ũ�ȣ�ϡ�ͺ����ʵ������䣬�����ݴ˼��㣻
��1����������Ὺʼû�����壬��������������ʣ�࣬Ӧ�����������ƺ�̼���ƵĻ����������������������ʣ������������Լ�������̼���Ƶ����������õ�̼���Ƶ������ʵ���������NaOH��Һ�����ʵ���Ũ�ȣ�
��2������̼Ԫ���غ��ϲ����Ķ�����̼�����Ķ��ټ���̼��ƺ�̼��þ�����������������ʵİٷֺ�����
��� �⣺��1������Ҫ�Ȼ�������ΪVL����HCl�����ʵ���Ϊ$\frac{V}{22.4}$mol����HCl������Ϊ$\frac{V}{22.4}$mol��36.5g/mol=$\frac{36.5V}{22.4}$g��1.00Lˮ������Ϊ1L��1000g/L=1000g������Һ������Ϊ��1000+$\frac{36.5V}{22.4}$��g���ʣ�1000+$\frac{36.5V}{22.4}$��g��36.5%=$\frac{36.5V}{22.4}$g����ã�V=352.8L��
�𣺽�352.8L�Ȼ��⣨��״����ͨ��1.00Lˮ�пɵõ�36.5%��Ũ���
��2����������Ϊ36.5%���ܶ�1.20g/cm3��Ũ��������ʵ���Ũ��Ϊ$\frac{1000��1.2��36.5%}{36.5}$mol/L=12.0mol/Lȡ10mL36.5%�����ᣬ��ˮϡ����300mL����ϡ�ͺ���������ʵ���Ũ����$\frac{12mol/L}{30}$=0.4mol/L����ϡ�ͺ���������ʵ���Ũ����0.4mol/L��
��1��NaOH+HCl=NaCl+H2O��Na2CO3+HCl=NaHCO3+NaCl��NaHCO3+HCl=CO2+NaCl+H2O���������ĵ�����������������Һ�������̼���ƺ�̼�����ƵĻ���
����Na2CO3+HCl=NaHCO3+NaCl��̼���Ƶ����ʵ����ǣ�0.4mol��0.02L=0.008mol��̼�������ɵ�̼���������ĵ���������ʵ�����0.008mol�������20mL�����������̼�����Ƶ����ʵ�����0.01L��0.4mol/L=0.004mol��������Ԫ���غ㣬�����������Ƶ����ʵ�����0.008��2+0.004mol=0.02mol�������������Ƶ����ʵ���Ũ��ʱ$\frac{0.02mol}{0.1L}$=0.2mol/L����500mL NaOH��Һ�����ʵ���Ũ����0.2mol/L��
��2������̼Ԫ���غ㣬C�����ʵ�����0.012mol��5=0.06mol����̼��ƺ�̼��þ�����ʵ����ֱ���x��y����x+y=0.06��100x+84y=5.78�����x=0.04��y=0.02������ԭ�������̼��Ƶ�����������$\frac{0.04��100}{5.68}$��100%=70.4%����ԭ�������̼��Ƶ�����������70.4%��
���� ������Ҫ�����йػ�ѧ����ʽ�ļ��㣬��Ŀ�ѶȽϴ�����̽ϸ��ӣ������Ĺؼ������жϳ���������ɣ�Ȼ���������ݼ�Ĺ�ϵ�����Ҫ������������ֿ�����ѧ���ķ���������������

 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�| A�� | ͨ��״���£�1 NA ��CO2����ռ�е����Ϊ22.4 L | |
| B�� | ��״���£�22.4 L H2O���еķ�����Ϊ1 NA | |
| C�� | ���³�ѹ�£�1.06 g Na2CO3���е�Na+��Ϊ0.02 NA | |
| D�� | ���ʵ���Ũ��Ϊ0.5 mol/L��MgCl2��Һ�У�����Cl- ����Ϊ1 NA |
| A�� | H2��g��+Cl2��g���T2HCl��g����H=-184.6kJ/mol | |
| B�� | CH4��g��+2O2��g���TCO2��g��+2H2O��g����H=-802.3kJ/mol | |
| C�� | 2H2��g��+O2��g���T2H2O��l����H=-571.6kJ/mol | |
| D�� | CO��g��+O2��g���TCO2��g����H=-258 kJ/mol |
| A�� | ��ij��Һ�У��ȼ�����ˮ�����KSCN��Һ������Һ�ʺ�ɫ����˵����Һ�к���Fe2+ | |
| B�� | �ò�˿պȡ����Һ���ڻ��������գ�������ʻ�ɫ����˵������Һ�к��������ӣ����������� | |
| C�� | ��Һʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ�����һ���ྻ���ձ��� | |
| D�� | ����ʱ��Ӧ���¶ȼƵ�ˮ�������������ƿ�ڵĻ��Һ���� |
 �������й�PHB��˵������ȷ���ǣ�������
�������й�PHB��˵������ȷ���ǣ�������| A�� | PHB�Ǹ߷��ӻ����� | |
| B�� | �ϳ�PHB�ĵ�����CH3CH2CH��OH��COOH | |
| C�� | ͨ�����۷�Ӧ�����Ƶ�PHB | |
| D�� | ��PHB�Ľ��������һ��û�������μӷ�Ӧ |
| A�� | ��Һ��c��Ca2+�������� | B�� | ��Һ��pH������ | ||
| C�� | ��Һ��Ca2+���������� | D�� | ��Һ��c��OH-����С |
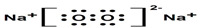
 X��Y��Z��W��N��ԭ���������������ǰ������Ԫ�أ�����X��s�ܼ���������p�ܼ���������2����Y��ԭ�Ӻ�����3��δ�ɶԵ��ӣ�Z�Ļ�̬ԭ��M����K���������ȣ�Y��Z������������֮�͵���W��������������N+ԭ�Ӻ�����3�����Ӳ��Ҹ��������ȫ��״̬���ش��������⣺
X��Y��Z��W��N��ԭ���������������ǰ������Ԫ�أ�����X��s�ܼ���������p�ܼ���������2����Y��ԭ�Ӻ�����3��δ�ɶԵ��ӣ�Z�Ļ�̬ԭ��M����K���������ȣ�Y��Z������������֮�͵���W��������������N+ԭ�Ӻ�����3�����Ӳ��Ҹ��������ȫ��״̬���ش��������⣺