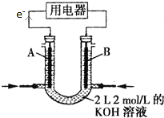
��Ŀ����
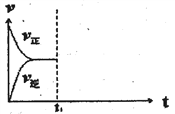
����Ŀ����ij�ܱ������м���0.3molA��0.1molC��һ������B�������壬һ�������·������·�Ӧ��3A(g)![]() B(g)+2C(g)�������ʵ�Ũ����ʱ��仯��ͼ��ʾ[t0��t1�ε�c(B)�仯δ����]������˵������ȷ����
B(g)+2C(g)�������ʵ�Ũ����ʱ��仯��ͼ��ʾ[t0��t1�ε�c(B)�仯δ����]������˵������ȷ����

A. ��t1=15s������A��Ũ�ȱ仯��ʾt0��t1�ε�ƽ����Ӧ����Ϊ0.004 mol/(L��s)
B. t1ʱ�÷�Ӧ�ﵽƽ�⣬A��ת����Ϊ40%
C. ���������ݻ�Ϊ2L��B����ʼ�����ʵ���Ϊ0.02 mol
D. t0��t1�Σ��˹����������������Ƚ�������Ϊa kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ3A(g)![]() B(g)+2C(g) ��H=-50a/3 kJ/mol
B(g)+2C(g) ��H=-50a/3 kJ/mol
���𰸡�D
��������A.����v����c/��t���㣻
B.����A����ʼ����ת��������ת���ʣ�
C.����B��ƽ�����ͱ仯�����㣻
D.����A����������������仯���㷴Ӧ�ȡ�
A.t0��t1�Σ�A��Ũ�ȱ仯Ϊ0.15mol/L-0.06mol/L=0.09 mol/L��t0��t1�ε�ƽ����Ӧ����Ϊ0.09mol/L��15s=0.006 mol��L��1��s��1��A����
B.t1ʱ�÷�Ӧ�ﵽƽ�⣬����ѡ��A�з�����֪A��ת����Ϊ0.09/0.15��100%=60%��B����
C.���ݷ�Ӧ3A(g)![]() B(g)��2C(g)��֪����Ӧ��ƽ����c(A)=0.09 mol��L��1�����c(B)=O.03 mol��L��1����ͼ���֪��Ӧ��ƽ���c(B)=0.05 mol��L��1������B����ʼ��Ũ��Ϊ0.02 mol��L��1��B����ʼ�����ʵ���Ϊ0.02mol/L��2L=0.04 mol��C����
B(g)��2C(g)��֪����Ӧ��ƽ����c(A)=0.09 mol��L��1�����c(B)=O.03 mol��L��1����ͼ���֪��Ӧ��ƽ���c(B)=0.05 mol��L��1������B����ʼ��Ũ��Ϊ0.02 mol��L��1��B����ʼ�����ʵ���Ϊ0.02mol/L��2L=0.04 mol��C����
D.t0��t1�Σ���c(A)=0.09 mol��L��1����n(A)=0.09mol/L��2L=0.18 mol����ʱ����a kJ�������3 mol A��ȫ��Ӧ������Ϊ50a/3 kJ�����Ȼ�ѧ����ʽΪ3A(g)![]() B(g)��2C(g) ��H����50a/3 kJ��mol��1��D��ȷ��
B(g)��2C(g) ��H����50a/3 kJ��mol��1��D��ȷ��
��ѡD��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��I������������Ӧ��
��Ӧ��![]()
![]() =a kJ/molƽ�ⳣ��ΪK1
=a kJ/molƽ�ⳣ��ΪK1
��Ӧ��![]()
![]() ƽ�ⳣ��ΪK2
ƽ�ⳣ��ΪK2
��Ӧ��![]()
![]() =b kJ/molƽ�ⳣ��ΪK3
=b kJ/molƽ�ⳣ��ΪK3
�ڲ�ͬ�¶��£�����K1��K2��ֵ���£�
T/�� | 700 | 800 |
K1 | 2.38 | 2.56 |
K2 | 0.80 |
(1) ![]()
![]() ==____________________
==____________________
(2)K1�ı���ʽΪ____________�����ݷ�Ӧ�����������Ƶ���K1��K2��K3�Ĺ�ϵʽK3=______________��
(3)�ں��º�ѹ�ܱ�������ͨ��CO��H2O��1mol������Ӧ�ڣ�����Ӧ�ﵽƽ���ά���¶���ѹǿ���䣬t1ʱ��ͨ���1mol��CO��H2O�Ļ�����壬������ͼ�л�������v�������棨v������Ӧ������t1����ʱ��t�仯������ͼ_______��
�����ݻ���ͬ�������ܱ�������(װ�е�����ij�ִ���)���ֱ����ͬ����NOx��C3H6���ڲ�ͬ�¶��£�ͬʱ�������·�Ӧ��
18NO(g)+2C3H6(g) 9N2(g)+6CO2(g)+6H2O(g)��
18NO2(g)+4C3H6(g) 9N2(g)+12CO2(g)+12H2O(g)��
���ֱ���t��ʱ�ⶨ����NOxת���ʣ����ͼ������ͼ��ʾ��
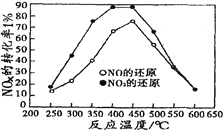
�ٴ�ͼ�п��Եó��Ľ�����
����һ���ӲⶨNOxת���������жϣ���ͬ�¶���NOת��Ч�ʱ�NO2�ĵ͡�
���۶���________________________________________________________
��������NO2��C3H6��Ӧ�У����NO2ת���ʵĴ�ʩ��_____________��(����)
A��������� B�������¶� C.�����H
���³�ѹ�£������е�CO2����ˮ���ﵽƽ��ʱ����Һ��pH=5.6��c(H2CO3)=1.5��10 -5 mol/L��������ˮ�ĵ��뼰H2CO3�ĵڶ������룬��H2CO3HCO3��+ H+ ��ƽ�ⳣ��K1=___________��(��֪��10-5.60=2.5��10-6 )