��Ŀ����
��12�֣���һ���¶��£���һ���ݻ�������ܱ������м��� 2 molA �� 2 molB ��������Ӧ��2A(g) + B(g) 3C( g) ��H����a kJ/mol��a��0�����ﵽƽ��ʱ�ų�������Ϊ bkJ��B���������Ϊ��1�����Իش�
3C( g) ��H����a kJ/mol��a��0�����ﵽƽ��ʱ�ų�������Ϊ bkJ��B���������Ϊ��1�����Իش�
��1���÷�Ӧƽ�ⳣ��K����ʽΪ________________________�� a��b�Ĺ�ϵ��a b�������������������=������
��2���������¶ȣ�ƽ�ⳣ��K__________�����������С�����䡱����ͬ�����ﵽƽ��ʱB���������_________��
��3������˵�����ܱ����÷�Ӧ�Ѵﵽƽ��״̬����_________��
��4����������ƽ����ϵ���ٳ���1 molB ��3 molC������ͬ�����´ﵽƽ��ʱB���������Ϊ��2 %�����1% ��2 % ����������� ������������������ ��
 3C( g) ��H����a kJ/mol��a��0�����ﵽƽ��ʱ�ų�������Ϊ bkJ��B���������Ϊ��1�����Իش�
3C( g) ��H����a kJ/mol��a��0�����ﵽƽ��ʱ�ų�������Ϊ bkJ��B���������Ϊ��1�����Իش���1���÷�Ӧƽ�ⳣ��K����ʽΪ________________________�� a��b�Ĺ�ϵ��a b�������������������=������
��2���������¶ȣ�ƽ�ⳣ��K__________�����������С�����䡱����ͬ�����ﵽƽ��ʱB���������_________��
��3������˵�����ܱ����÷�Ӧ�Ѵﵽƽ��״̬����_________��
| A���¶Ⱥ����һ��ʱ��������ѹǿ���ٱ仯 |
| B���¶Ⱥ����һ��ʱ��ijһ����Ũ�Ȳ��ٱ仯 |
| C������һ������������ƽ����Է����������ٱ仯 |
| D���¶Ⱥ�ѹǿһ��ʱ�����������ܶȲ��ٱ仯 |
��1��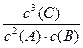 �� 2�֣���> �� 2�֣� (2) ��С��1�֣��� ����1�֣�
�� 2�֣���> �� 2�֣� (2) ��С��1�֣��� ����1�֣�
��3��B ��2�֣�
��4��=�� 2�֣��� ��÷�ӦΪ��Ӧǰ���������������ķ�Ӧ������ͬ���������ܱ������С����� 2 molA �� 2 molB���롰���� 1 molB �� 3 molC��Ϊ��Чƽ�⡣��2�֣�
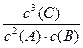 �� 2�֣���> �� 2�֣� (2) ��С��1�֣��� ����1�֣�
�� 2�֣���> �� 2�֣� (2) ��С��1�֣��� ����1�֣���3��B ��2�֣�
��4��=�� 2�֣��� ��÷�ӦΪ��Ӧǰ���������������ķ�Ӧ������ͬ���������ܱ������С����� 2 molA �� 2 molB���롰���� 1 molB �� 3 molC��Ϊ��Чƽ�⡣��2�֣�
��

��ϰ��ϵ�д�
�����Ŀ
 2C3(g)����H=��Q1 kJ��mol(Q1>0)����һ���д����Ĺ̶�
2C3(g)����H=��Q1 kJ��mol(Q1>0)����һ���д����Ĺ̶�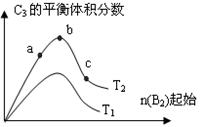
 00��ʱ��ַ�Ӧ����ƽ���C3��Ũ��Ϊ
00��ʱ��ַ�Ӧ����ƽ���C3��Ũ��Ϊ �����������Q3
�����������Q3 kJ��C3Ũ�� (��>��=��<)w mol/L��Q1��Q2��Q3֮��������ֹ�ϵ (�ô���ʽ��ʾ)��
kJ��C3Ũ�� (��>��=��<)w mol/L��Q1��Q2��Q3֮��������ֹ�ϵ (�ô���ʽ��ʾ)�� ��(��Ӧǰ�����ͬ)����ʼʱ����2 molA2��l molB2��500��ʱ��ַ�Ӧ��ƽ��ų�����Q4 kJ����Q2 Q4 ( �� > �� = �� < )�������� ��
��(��Ӧǰ�����ͬ)����ʼʱ����2 molA2��l molB2��500��ʱ��ַ�Ӧ��ƽ��ų�����Q4 kJ����Q2 Q4 ( �� > �� = �� < )�������� ��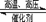 2NH3����H<0������N2��H2��1�U1�����ʵ���֮�Ȼ��Ⱥ�ֳ����ȷݣ�ͬʱ�ֱ����A�����B�������װ�д�������������У�A��B�������ݻ��̶������ڱ���ͬ�¶��£�A��B���������ĺϳɰ���Ӧ�Ⱥ�ﵽƽ��״̬����ش�
2NH3����H<0������N2��H2��1�U1�����ʵ���֮�Ȼ��Ⱥ�ֳ����ȷݣ�ͬʱ�ֱ����A�����B�������װ�д�������������У�A��B�������ݻ��̶������ڱ���ͬ�¶��£�A��B���������ĺϳɰ���Ӧ�Ⱥ�ﵽƽ��״̬����ش� _KB���>������<����=������
_KB���>������<����=������ ʩΪ__________��
ʩΪ__________�� ʱ������ƽ����Է�������]����ƽ��ʱ������A��N2��ת����Ϊ___________������B��M��ƽ��Ϊ__________������______���A����B�����У�NH3�����ʵ�����ռ
ʱ������ƽ����Է�������]����ƽ��ʱ������A��N2��ת����Ϊ___________������B��M��ƽ��Ϊ__________������______���A����B�����У�NH3�����ʵ�����ռ �ı����ϴ�
�ı����ϴ�
 PCl3(g)+Cl2(g) ��H��0 K��1 ��
PCl3(g)+Cl2(g) ��H��0 K��1 ��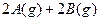

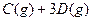 ��
��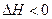 ���ֽ�
���ֽ�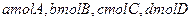 �������ΪV��ij�����У���ƽ��ʱ�����ʵ�Ũ��ǡ����ȣ�������һ���¶Ⱥ�Ӧ�ﵽ��ƽ��ʱ�Ļ�ƽ�ⳣ������Ϊ�� ��
�������ΪV��ij�����У���ƽ��ʱ�����ʵ�Ũ��ǡ����ȣ�������һ���¶Ⱥ�Ӧ�ﵽ��ƽ��ʱ�Ļ�ƽ�ⳣ������Ϊ�� �� pC(g)(����ӦΪ���ȷ�Ӧ)�Ŀ��淴Ӧ���ں��������£�ƽ��ʱB�ڻ�����еĺ�����B%����ѹǿ�Ĺ�ϵ��ͼ2��32��ʵ����ʾ���й�������ȷ���� �� ��
pC(g)(����ӦΪ���ȷ�Ӧ)�Ŀ��淴Ӧ���ں��������£�ƽ��ʱB�ڻ�����еĺ�����B%����ѹǿ�Ĺ�ϵ��ͼ2��32��ʵ����ʾ���й�������ȷ���� �� ��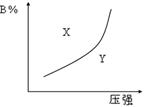
 cC��g��+dD��s��������Ӧ����һ��ʱ����A������nmol��B������n��2mol��C������3n��2mol��D������nmol����ʱ�ﵽ��ѧƽ��״̬��������˵����ȷ���� �� ��
cC��g��+dD��s��������Ӧ����һ��ʱ����A������nmol��B������n��2mol��C������3n��2mol��D������nmol����ʱ�ﵽ��ѧƽ��״̬��������˵����ȷ���� �� ��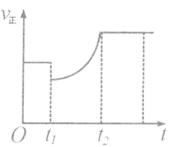
 ����ʱ��ı仯�����t1ʱ�̿����Ǽ�����A��Ũ�ȣ�������C��Ũ��
����ʱ��ı仯�����t1ʱ�̿����Ǽ�����A��Ũ�ȣ�������C��Ũ�� 2HI(g)�����������ܹ�˵����Ӧ�Ѵ�ƽ��״̬����
2HI(g)�����������ܹ�˵����Ӧ�Ѵ�ƽ��״̬���� HX��g����ƽ�ⳣ��Ϊ9������2.0 mol
HX��g����ƽ�ⳣ��Ϊ9������2.0 mol