��Ŀ����
19��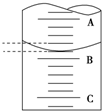 ʵ������һƿ�����Ȼ��Ƶ��������ƹ����Լ������ⶨ�������Ƶ���������ԼΪ82%��Ϊ����֤�䴿�ȣ���Ũ��Ϊ0.2mol•L-1��������еζ�������������⣺
ʵ������һƿ�����Ȼ��Ƶ��������ƹ����Լ������ⶨ�������Ƶ���������ԼΪ82%��Ϊ����֤�䴿�ȣ���Ũ��Ϊ0.2mol•L-1��������еζ�������������⣺��1����ȡ5.0g���������ƹ�����Ʒ�����500mL��Һ���ã�
��2����������װ��25.00mL����ʽ�ζ����У�����Һ��λ���ڡ�0���̶����£�����¼�¿̶ȣ�
��3��ȡ20.00mL����Һ������ʵ�����ʹ�õ���Ҫ�����м�ʽ�ζ��ܡ���ƿ���÷�̪��ָʾ��ʱ���ζ�����Һ��ɫ�ɺ�ɫɫ�պñ����ɫΪֹ��
��4���ζ����յ����������ȥ20.00mL�������������Ƶ���������Ϊ80.0%��
��5���Է��������ζ���������������Щʵ���������ACE������ţ���
A��ת�ƴ���Һ������ƿʱ��δϴ���ձ�
B����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ����
C���ζ�ʱ��Ӧ��ҡ��̫���ң�������Һ�彦��
D���ζ����յ�ʱ���ζ��ܼ�����������
E���ζ���ʼʱ�������ӣ��յ�ʱ��������
��6����ͼ��ʾ50.00mL�ζ�����Һ���λ�ã���A��C�̶ȼ����1mL��A���Ŀ̶�Ϊ25.00mL���ζ�����Һ�����ӦΪ25.40mL�����ʱҺ���������Ϊa mL���ζ�����Һ������V���ڣ�50-a�� mL���=������������������
���� ��2������Ӧ����ʽ�ζ�����ȡ��
��3������ҺΪNaOH��ѡ���ʽ�ζ��ܻ���Һ�ܣ��ü�����ָʾ��ʱ���ζ�ǰ�����������ƣ��ζ��������ɫ��ֹͣ�ζ���
��4��c��HCl��=0.2mol/L��V��HCl��=20.00mL��V��NaOH��=20.00mL����c���ᣩV���ᣩ=c���V�������c��NaOH������һ������500mL��Һ��NaOH�����ʵ�����������Ʒ������5.0g��������������
��5������c�����⣩=$\frac{V��������c������}{V�����⣩}$��������������V��������Ӱ�죬�Դ��ж�Ũ�ȵ���
��6��A��C�̶ȼ����1mL��˵��ÿ����С��֮����0.1mL��A���Ŀ̶�Ϊ25.00mL���ݴ�ȷ��B�Ŀ̶ȣ�ע��ζ��ܵ�������ֵС��������ֵ�ζ��ܻ����·�����Ƥ���̶ȣ�
��� �⣺��2������Ӧ����ʽ�ζ�����ȡ���ʴ�Ϊ����ʽ��
��3������ҺΪNaOH��ѡ���ʽ�ζ��ܣ�������ƿ�У��÷�̪��ָʾ��ʱ���ζ�ǰ����̪�����죬����ζ��յ�ʱ�۲쵽��Һ��ɫ�ɺ�ɫ��Ϊ��ɫ��
�ʴ�Ϊ����ʽ�ζ��ܡ���ƿ����ɫ���ޣ�
��4��c��HCl��=0.2mol•L-1��V��HCl��=20.00mL��V��NaOH��=20.00mL����c���ᣩV���ᣩ=c���V�����֪c��NaOH��=0.2mol/L��
��500mL��Һ��NaOH�����ʵ���Ϊ0.5L��0.2mol/L=0.1mol����NaOH������Ϊ0.1mol��40g/mol=4.0g������5.0g��Ʒ��NaOH����������Ϊ$\frac{4.0g}{5.0g}$��100%=80.0%��
�ʴ�Ϊ��80.0%��
��5��A��ת�ƴ���Һ������ƿʱ��δϴ���ձ���NaOH�����ʵ������٣�����V���ᣩ���٣�����c�����⣩=$\frac{V��������c������}{V�����⣩}$������c�����⣩ƫ�ͣ����������Ƶ���������ƫ�ͣ���A��ȷ��
B����ʽ�ζ���������ˮϴ�Ӻ�ֱ��װ���ᣬ�����Ũ��ƫ�ͣ�����V���ᣩƫ����c�����⣩=$\frac{V��������c������}{V�����⣩}$������c�����⣩ƫ�����������Ƶ���������ƫ�ߣ���B����
C���ζ�ʱ����Ӧ����ҡ��̫���ң�������Һ�彦����NaOH�����ʵ������٣�����V���ᣩ���٣�����c�����⣩=$\frac{V��������c������}{V�����⣩}$������c�����⣩ƫ�ͣ����������Ƶ���������ƫ�ͣ���C��ȷ��
D���ζ����յ�ʱ���ζ��ܼ�������Һ�Σ�����V���ᣩƫ����c�����⣩=$\frac{V��������c������}{V�����⣩}$������c�����⣩ƫ�����������Ƶ���������ƫ�ߣ���D����
E������ʽ�ζ��ܿ�ʼʱ���ӣ�����ƫ�����յ�ʱ���ӣ�����ƫС������֮��ƫС������V���ᣩ���٣�����c�����⣩=$\frac{V��������c������}{V�����⣩}$������c�����⣩ƫ�ͣ����������Ƶ���������ƫ�ͣ���E��ȷ��
�ʴ�Ϊ��ACE��
��6��A��C�̶ȼ����1mL��˵��ÿ����С��֮����0.10mL��A���Ŀ̶�Ϊ25.00mL��A��B֮�����ĸ�С���������0.40mL����B��25.40mL���ζ��̶ܿ�ֵ���ϵ��¿̶����������ڵζ��ܻ����·�����Ƥ���̶ȣ�50mL�ζ�����ʵ��ʢ��Һ����������50mL�����Һ�洦�Ķ�����a����ζ�����Һ���������ڣ�50-a��mL��
�ʴ�Ϊ��25.40�����ڣ�
���� ���⿼���к͵ζ������յζ�ԭ����ʵ��������������Ϊ���Ĺؼ���ע�����������ü��㹫ʽ���ᡢ����������Ŀ�ѶȲ���

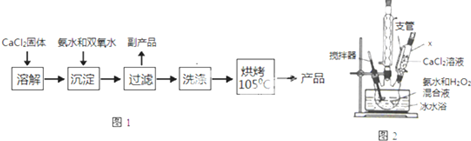
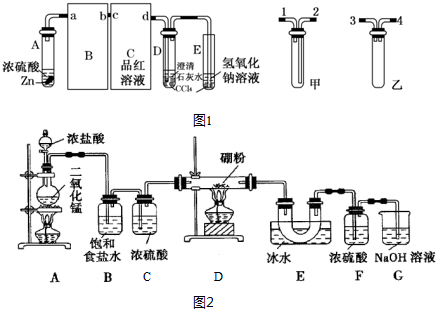
����CaO2���Ʊ�ԭ����CaCl2+H2O2+2NH3•H2O+6H2O�TCaO2•8H2O��+2NH4Clʵ�鲽����ͼ1����Ӧװ����ͼ2��ʾ����ش��������⣺
��1������x������Ϊ��ѹ��Һ©�������ѹ��Һ©����
��2����ƽ���ƶ�ԭ�����ͼ��백ˮ���������кͷ�Ӧ���ɵ�HCl��ʹCaCl2+H2O2?CaO2+2HCl���ҽ��У�
��3��������Ӧʱ���ñ�ˮԡ�����¶���0�����ң������ԭ���Ǽ���˫��ˮ���ȷֽ⡢���Ͳ����ܽ�ȱ���������д�����֣�
��4�����˺�ϴ�ӳ������Լ������B
A����ˮ����B����ˮ������C���Ҵ�������D������
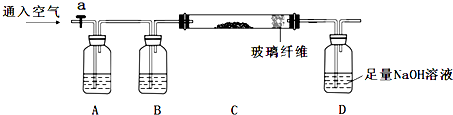
����CaO2���ȼ�⣬��һ����CaO2����ϡ���ᣬ�ñ�KMnO4���ڵζ����ɵ�H2O2��KMnO4��Ӧ������Mn2+������ȷ��CaO2�ĺ�����
��5����ÿ�γ�ȡ0.4000g��Ʒ�ܽ����0.1000mol/L��KMnO4��Һ�ζ��������������ʾ����CaO2��Ʒ�Ĵ���90.00%
| ʵ����� | ��1�� | ��2�� | ��3�� | ��4�� |
| ����KMnO4���/mL | 19.98 | 20.02 | 20.20 | 20.00 |
A���濾ʱ�䲻��
B���ڽྻ�������ʽ�ζ�����δ��ϴ��װ��Һ
C���ζ�ǰ���촦�����ݣ��ζ�����ʧ
D������KMnO4����Һ����ʱ��������ƿ���ߣ�
| A�� | ̼ | B�� | �� | C�� | �� | D�� | �� |
| A�� | C2H2 | B�� | BeCl2 | C�� | CO2 | D�� | HClO |
 ��ͼΪп��ͭ������ͭ��Һ���ɵ�ԭ��أ���д���������Լ�����ܷ�Ӧ��
��ͼΪп��ͭ������ͭ��Һ���ɵ�ԭ��أ���д���������Լ�����ܷ�Ӧ��