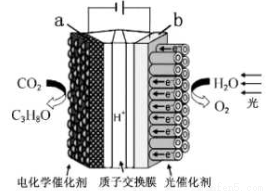
��Ŀ����
13��ijͬѧ��18mol/L��Ũ���� ����240mL 0.9mol/L��ϡ���ᣬ�������й�ʵ�飮��ش��������⣺��1����Ҫ��ȡŨ����12.5mL��
��2����������Ʒ��ѡ��ʵ������Ҫ������ΪBCFI������ţ���
A��500mL�ձ��� B��100mL�ձ��� C��25mL��Ͳ�� D��100mL��Ͳ��
E��500mL����ƿ�� F��250mL����ƿ�� G�����ƿ�� H��������ƽ��
I��������
��ѡ�����������⣬��ȱ�ٵı�Ҫ����������Ʒ�ǽ�ͷ�ιܣ�
��3�������ƹ����У�������ʱ���ӿ̶��ߣ���������ҺŨ�ȴ���0.9mol•L-10.9mol•L-1������ڡ��������ڡ���С�ڡ�����
��4��ȡ�����Ƶ�ϡ����100mL����һ��������п��ַ�Ӧ��пȫ���ܽ�����ɵ������ڱ�״���µ����Ϊ0.448L����μӷ�Ӧ��п������Ϊ1.3g���跴Ӧ����Һ�������Ϊ100mL����Ӧ����Һ��H+�����ʵ���Ũ��Ϊ1.4mol/L��
���� ��1������������Һ�����ѡ����ʵ�����ƿ��������Һϡ�������������ʵ����ʵ������������ҪŨ���������
��2��������Ũ��Һ��һ�����ʵ���Ũ��ϡ��Һ�IJ�������ѡȡʵ��������
��3�������ƹ����У�������ʱ���ӿ̶��ߵ�����Һ���ƫС������C=$\frac{n}{V}$���з�����
��4�����ݷ���ʽ����μӷ�Ӧ��п����������ʵ���������m=nM����п����������Һ��n��H+��=n��H2SO4�����������������ʵ���Ũ�ȱ仯������������Ũ�ȱ仯���������ӿ�ʼŨ�ȼ�ȥ������Ũ�ȱ仯�����ڷ�Ӧ����Һ�е������ӵ����ʵ���Ũ�ȣ�
��� �⣺��1������240mL 0.9mol/L��ϡ���ᣬ��Ҫѡ��250mL����ƿ������ҪŨ�������ΪV����������Һϡ�������������ʵ����ʵ�������ã�V��18mol/L=0.9mol/L��0.25L�����V=0.0125L��
�ʴ�Ϊ��12.5��
��2������Ũ��Һ��һ�����ʵ���Ũ��ϡ��Һ�IJ�������Ϊ�����㡢��ȡ��ϡ�͡���Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ������õ���������B��100mL�ձ���C��25mL��Ͳ��F��250mL����ƿ��I����������
��ȱ�ٵ�����Ϊ����ͷ�ιܣ�
�ʴ�Ϊ��BCFI����ͷ�ιܣ�
��3�������ƹ����У�������ʱ���ӿ̶��ߵ�����Һ���ƫС������C=$\frac{n}{V}$��֪����ҺŨ��ƫ�ߣ�
�ʴ�Ϊ������0.9mol•L-1��
��4���������������$\frac{0.448L}{22.4L/mol}$=0.02mol��
Zn+H2SO4=ZnSO4+H2��
1mol 1mol 1mol
x y 0.02mol
����x=0.02mol��
y=0.02mol��
���Բμӷ�Ӧп������Ϊ0.02mol��65g/mol=1.3g��
������Ũ�ȱ仯��Ϊ��c��H+��=$\frac{0.02mol��2}{0.1L}$=0.4mol/L��
���Է�Ӧ����Һ�е������ӵ����ʵ���Ũ��Ϊ2��0.9mol/L-0.4mol/L=1.4mol/L��
�ʴ�Ϊ��1.3g��1.4mol/L��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ��йط���ʽ�ļ��㣬��ȷ����ԭ����������������ķ����ǽ���ؼ�����Ŀ�ѶȲ���

| A�� | ��Һ�ͽ�������ö����ЧӦ���� | |
| B�� | ��NaOH��Һ��FeCl3��Һ��Ͽ��Ƴ�Fe��OH��3���� | |
| C�� | ��������Һ��������Һ�������dz����Ľ��� | |
| D�� | ��Һ���������Һ�ı��������Ƿ�ɢ����ֱ���Ĵ�С |
| A�� | ̼��þ��H2SO4��Ӧ MgCO3+2H+=Mg2++H2O+CO2�� | |
| B�� | ����������Һ�м����Ag++Cl-=AgCl�� | |
| C�� | ����ϡ���ᷴӦ 2Fe+6H+=2Fe3++3H2�� | |
| D�� | ����ʯ��ˮ������ķ�Ӧ H++OH-=H2O |
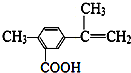
| A�� | ���л���ķ���ʽΪC11H12O2 | |
| B�� | 1mol�������������4molH2�����ӳɷ�Ӧ | |
| C�� | ���л���һ�������£����Է���ȡ����������������Ӧ | |
| D�� | ���л��������ȵ�����������ͭ����Һ��Ӧ������ש��ɫ���� |


 CH3COOCH2CH3+H2O������������ȡ������Ӧ��
CH3COOCH2CH3+H2O������������ȡ������Ӧ��