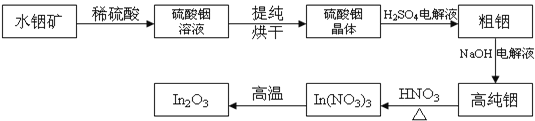
МвДїДЪИЭ
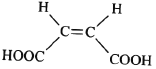
ЎѕМвДїЎїСхµЄФУКЗРВТ©СРЦЖ№эіМЦР·ўПЦµДТ»АаЦШТЄ»оРФОпЦКЈ¬ДЬУГУЪёДЙЖґуДФИ±СЄЎЈПВГжКЗДіСРѕїРЎЧйМбіцµДТ»ЦЦСхµЄФУАа»ЇєПОпEµДєПіЙВ·ПЯЈє
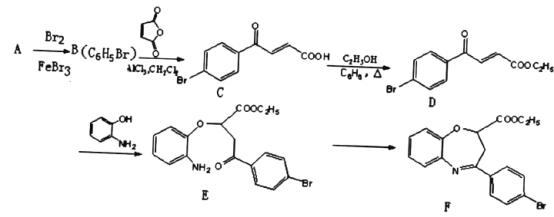
ЈЁ1Ј©AµДЅб№№јтКЅОЄ_____ЎЈCЦР№ЩДЬНЕµДГыіЖОЄ____ЎЈ
ЈЁ2Ј©EЎъFѕАъБЅІЅ·ґУ¦Ј¬Жд·ґУ¦АаРНТАґООЄ______Ўў______ЎЈ
ЈЁ3Ј©Н¬К±ВъЧгПВБРМхјюµДDµДТ»ЦЦН¬·ЦТм№№МеµДЅб№№јтКЅОЄ______ЎЈ
I.·ЦЧУЦРє¬УР1ёц±Ѕ»·Ј»
II.ДЬ·ўЙъТшѕµ·ґУ¦Ј»
III.·ЦЧУЦРУР5ЦЦІ»Н¬»ЇС§»·ѕіµДЗвЎЈ
ЈЁ4Ј©1mol DУлЧгБїNaOHИЬТє·ґУ¦К±Чо¶аПыєД_____molNaOHЎЈ
ЈЁ5Ј©УЙEєПіЙFК±»№їЙДЬЙъіЙё±ІъОп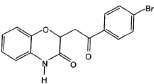 єНТ»ЦЦіЈјыµДУР»ъОпGЈ¬GµДЅб№№јтКЅОЄ_______ЎЈ
єНТ»ЦЦіЈјыµДУР»ъОпGЈ¬GµДЅб№№јтКЅОЄ_______ЎЈ
ЈЁ6Ј©ТСЦЄЈє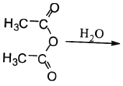 CH3COOHЎЈФБП
CH3COOHЎЈФБП![]() ЛЧГыЎ°ВнАіфыЎ±Ј¬ЛьКЗВнАіЛб
ЛЧГыЎ°ВнАіфыЎ±Ј¬ЛьКЗВнАіЛб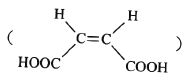 µДЛбфыЎЈЗлРґіцТФ
µДЛбфыЎЈЗлРґіцТФ![]() ОЄФБПЦЖ±ё»ЇєПОп
ОЄФБПЦЖ±ё»ЇєПОп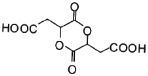 µДєПіЙВ·ПЯБчіМНј(ОЮ»ъКФјБИОУГ)ЎЈ______
µДєПіЙВ·ПЯБчіМНј(ОЮ»ъКФјБИОУГ)ЎЈ______
єПіЙВ·ПЯБчіМНјКѕАэИзПВЈєCH3CH2OH![]() CH2=CH2
CH2=CH2![]() Br-CH2CH2-Br
Br-CH2CH2-Br
Ўѕґр°ёЎї![]() фК»щЎўфИ»щЎўМјМјЛ«јьЎўдеФЧУ јУіЙ·ґУ¦ ПыИҐ·ґУ¦
фК»щЎўфИ»щЎўМјМјЛ«јьЎўдеФЧУ јУіЙ·ґУ¦ ПыИҐ·ґУ¦ 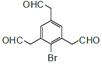 »т
»т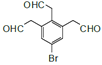 3 CH3CH2OH
3 CH3CH2OH ![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
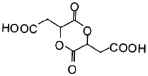
ЎѕЅвОцЎї
BОЄC6H5BrЈ¬ОЄде±Ѕ(![]() )Ј¬AУлде·ґУ¦ЙъіЙде±ЅЈ¬ФтAОЄ±Ѕ(
)Ј¬AУлде·ґУ¦ЙъіЙде±ЅЈ¬ФтAОЄ±Ѕ(![]() )Ј»ЅбєПБчіМНјЦРёчОпЦКµДЅб№№јтКЅєНЧЄ»Ї№ШПµ·ЦОцЅвґр(1)~(5)Ј»
)Ј»ЅбєПБчіМНјЦРёчОпЦКµДЅб№№јтКЅєНЧЄ»Ї№ШПµ·ЦОцЅвґр(1)~(5)Ј»
(6)ТЄєПіЙ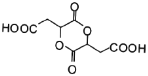 Ј¬їЙТФКЧПИєПіЙ
Ј¬їЙТФКЧПИєПіЙ![]() Ј¬їЙТФЅ«
Ј¬їЙТФЅ«![]() Л®ЅвЙъіЙ
Л®ЅвЙъіЙ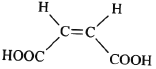 Ј¬
Ј¬ Улде»ЇЗвјУіЙЈ¬ЙъіЙ
Улде»ЇЗвјУіЙЈ¬ЙъіЙ![]() Ј¬
Ј¬![]() Л®ЅвЎўЛб»ЇЎўхҐ»ЇїЙµГЧоЦХІъЖ·
Л®ЅвЎўЛб»ЇЎўхҐ»ЇїЙµГЧоЦХІъЖ· Ј¬ѕЭґЛ·ЦОцЅвґрЎЈ
Ј¬ѕЭґЛ·ЦОцЅвґрЎЈ
(1)ёщѕЭЙПКц·ЦОцЈ¬AОЄ±Ѕ(![]() )Ј»C(
)Ј»C( )ЦР№ЩДЬНЕУРфК»щЎўфИ»щЎўМјМјЛ«јьЎўдеФЧУЈ¬№Кґр°ёОЄЈє
)ЦР№ЩДЬНЕУРфК»щЎўфИ»щЎўМјМјЛ«јьЎўдеФЧУЈ¬№Кґр°ёОЄЈє![]() Ј»фК»щЎўфИ»щЎўМјМјЛ«јьЎўдеФЧУЈ»
Ј»фК»щЎўфИ»щЎўМјМјЛ«јьЎўдеФЧУЈ»
(2)ёщѕЭБчіМНјЈ¬E(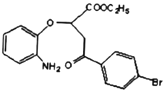 )ЎъF(
)ЎъF( )ѕАъБЅІЅ·ґУ¦Ј¬°±»щУлфК»щПИјУіЙЈ¬ФЩНСИҐТ»·ЭЧУЛ®Ј¬ЛщТФЖд·ґУ¦АаРН·Ц±рОЄјУіЙ·ґУ¦єНПыИҐ·ґУ¦Ј¬№Кґр°ёОЄЈєјУіЙ·ґУ¦Ј»ПыИҐ·ґУ¦Ј»
)ѕАъБЅІЅ·ґУ¦Ј¬°±»щУлфК»щПИјУіЙЈ¬ФЩНСИҐТ»·ЭЧУЛ®Ј¬ЛщТФЖд·ґУ¦АаРН·Ц±рОЄјУіЙ·ґУ¦єНПыИҐ·ґУ¦Ј¬№Кґр°ёОЄЈєјУіЙ·ґУ¦Ј»ПыИҐ·ґУ¦Ј»
(3)DОЄ ЎЈI.·ЦЧУЦРє¬УР1ёц±Ѕ»·Ј»II.ДЬ·ўЙъТшѕµ·ґУ¦Ј¬Ѕб№№ЦРє¬УРИ©»щЈ»III.·ЦЧУЦРУР5ЦЦІ»Н¬»ЇС§»·ѕіµДЗвЈ¬·ыєПЙПКцМхјюµДDµДТ»ЦЦН¬·ЦТм№№МеОЄ
ЎЈI.·ЦЧУЦРє¬УР1ёц±Ѕ»·Ј»II.ДЬ·ўЙъТшѕµ·ґУ¦Ј¬Ѕб№№ЦРє¬УРИ©»щЈ»III.·ЦЧУЦРУР5ЦЦІ»Н¬»ЇС§»·ѕіµДЗвЈ¬·ыєПЙПКцМхјюµДDµДТ»ЦЦН¬·ЦТм№№МеОЄ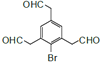 »т
»т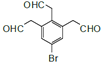 Ј¬№Кґр°ёОЄЈє
Ј¬№Кґр°ёОЄЈє »т
»т Ј»
Ј»
(4)1mol D( )ЦРє¬УР1molдеФЧУЈ¬1molхҐ»щЈ¬деФЧУЛ®ЅвЙъіЙµДфЗ»щОЄ·УфЗ»щЈ¬ТІДЬУлЗвСх»ЇДЖ·ґУ¦Ј¬ТтґЛ1mol DУлЧгБїNaOHИЬТє·ґУ¦К±Чо¶аПыєД3molNaOHЈ¬№Кґр°ёОЄЈє3Ј»
)ЦРє¬УР1molдеФЧУЈ¬1molхҐ»щЈ¬деФЧУЛ®ЅвЙъіЙµДфЗ»щОЄ·УфЗ»щЈ¬ТІДЬУлЗвСх»ЇДЖ·ґУ¦Ј¬ТтґЛ1mol DУлЧгБїNaOHИЬТє·ґУ¦К±Чо¶аПыєД3molNaOHЈ¬№Кґр°ёОЄЈє3Ј»
(5)УЙE ( )єПіЙF(
)єПіЙF( )К±Ј¬ёщѕЭё±ІъОп
)К±Ј¬ёщѕЭё±ІъОп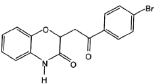 µДЅб№№їЙЦЄЈ¬EЦРхҐ»щЛ®ЅвЙъіЙТТґјЈ¬јґGµДЅб№№јтКЅОЄCH3CH2OHЈ¬№Кґр°ёОЄЈєCH3CH2OHЈ»
µДЅб№№їЙЦЄЈ¬EЦРхҐ»щЛ®ЅвЙъіЙТТґјЈ¬јґGµДЅб№№јтКЅОЄCH3CH2OHЈ¬№Кґр°ёОЄЈєCH3CH2OHЈ»
(6)ТФ![]() ОЄФБПЦЖ±ё»ЇєПОп
ОЄФБПЦЖ±ё»ЇєПОп Ј¬ТЄєПіЙ
Ј¬ТЄєПіЙ Ј¬їЙТФКЧПИєПіЙ
Ј¬їЙТФКЧПИєПіЙ![]() Ј¬їЙТФЅ«
Ј¬їЙТФЅ«![]() Л®ЅвЙъіЙ
Л®ЅвЙъіЙ Ј¬
Ј¬ Улде»ЇЗвјУіЙЈ¬ЙъіЙ
Улде»ЇЗвјУіЙЈ¬ЙъіЙ![]() Ј¬
Ј¬![]() Л®ЅвЎўЛб»ЇЎўхҐ»ЇїЙµГЧоЦХІъЖ·
Л®ЅвЎўЛб»ЇЎўхҐ»ЇїЙµГЧоЦХІъЖ· Ј¬ТтґЛєПіЙВ·ПЯОЄЈє
Ј¬ТтґЛєПіЙВ·ПЯОЄЈє![]()
![]()

![]()
![]()
![]()
![]()
![]()
![]()
![]()
 Ј¬№Кґр°ёОЄЈє
Ј¬№Кґр°ёОЄЈє![]()
![]()

![]()
![]()
![]()
![]()
![]()
![]()
![]()
 ЎЈ
ЎЈ

 ФД¶БїміµПµБРґр°ё
ФД¶БїміµПµБРґр°ёЎѕМвДїЎїўс.ПВНјКЗФЄЛШЦЬЖЪ±нµДТ»Ії·ЦЈ¬ёщѕЭўЩЎ«ўаФЪЦЬЖЪ±нЦРµДО»ЦГ°ґМвДїТЄЗу»ШґрЈє
Че ЦЬЖЪ | ўсA | ўтA | ўуA | ўфA | ўхA | ўцA | ўчA | 0 |
¶ю | ўЩ | ўЪ | ўЫ | |||||
Иэ | ўЬ | ўЭ | ўЮ | ўЯ | ўа |
(1)ФЄЛШўЩЎ«ўаЦРЈ¬іэўаНвЈ¬ФЧУ°лѕ¶ЧоґуµДКЗ___________ЈЁМоФЄЛШ·ыєЕЈ©Ј¬ФЄЛШўЫЎўўЯЛщРОіЙµДЗв»ЇОпµДОИ¶ЁРФУЙЗїµЅИхµДЛіРтОЄ__________________ЈЁУГПаУ¦Зв»ЇОпµД»ЇС§КЅЧчґрЈ©ФЄЛШўЪµДЗв»ЇОпµДµзЧУКЅКЗ____________ЎЈ
(2)ўЬўЭўЮИэЦЦФЄЛШµДЗвСх»ЇОпµДјоРФУЙЗїµЅИхµДЛіРтКЗ________ЈЁУГПаУ¦ЗвСх»ЇОпµД»ЇС§КЅЧчґрЈ©Ј¬ФЄЛШўЩЎ«ўаЦРµДЧоёЯјЫСх»ЇОп¶ФУ¦µДЛ®»ЇОпЦРЛбРФЧоЗїµДЛбКЗ_______(Мо»ЇС§КЅ)ЎЈ
ўт.КµСйКТУГГЬ¶ИОЄ1.84g/cm3Ј¬ИЬЦКµДЦКБї·ЦКэОЄ98%µДБтЛбЈ¬ЕдЦЖ980mLОпЦКµДБїЕЁ¶ИОЄ0.1mol/LµДБтЛбЎЈ№©СЎУГµДТЗЖчУРЈєўЩЅєН·µО№ЬЈ»ўЪТ©іЧЈ»ўЫЙХ±Ј»ўЬБїНІЈ»ўЭНРЕММмЖЅЎЈЗл»ШґрПВБРОКМвЈє
(1)ЕдЦЖЙПКцИЬТєБїИЎЕЁБтЛбК±У¦СЎУГ__________БїНІЈЁСЎМоРтєЕЈ©
ўЩ10mL ўЪ50mL ўЫ100mLЈ»
(2)ЕдЦЖПЎБтЛбК±Ј¬ЙПКцТЗЖчЦР»№И±ЙЩµДТЗЖчУР____________________________ЈЁРґТЗЖчГыіЖЈ©Ј¬І»РиТЄК№УГµДУР______________ЈЁСЎМоРтєЕЈ©Ј»
(3)ПВБРІЩЧчК№ЛщЕдИЬТєµДОпЦКµДБїЕЁ¶ИЖ«µНµДКЗ___________ЎЈ
A.УГБїНІБїИЎµДЕЁБтЛбЈ¬ВэВэµШСШЧЕІЈБ§°фЧўИлКўУР20mLХфБуЛ®µДРЎЙХ±Ј¬ЅБ°иєуЈ¬БўјґЧЄТЖµЅИЭБїЖїЦРЈ¬ФЩ°ґХэИ·ІЩЧчЕдЦЖИЬТєЎЈ
B.НщИЭБїЖїЧЄТЖИЬТєК±Ј¬УРЙЩБїТєМеЅ¦іцЎЈ
C.ОґПґµУПЎКНЕЁБтЛбµДРЎЙХ±
D.¶ЁИЭК±ё©КУїМ¶ИПЯЎЈ
E.ИЭБїЖїОґёЙФпјґУГАґЕдЦЖИЬТєЎЈ
F.¶ЁИЭєуИыЙПЖїИы·ґёґТЎФИЈ¬ѕІЦГєуЈ¬ТєГжІ»µЅїМ¶ИПЯЎЈ