��Ŀ����
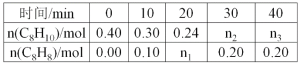
����Ŀ��������(C8H10)���������ϵ��屽��ϩ(C8H8)���䷴Ӧԭ���ǣ�C8H10(g)![]() C8H8(g)+H2(g)��H=120kJmol-1ij�¶��£���0.40mol�����飬����2L����ܱ������з�����Ӧ���ⶨ��ͬʱ����������������ʵ������õ��������±���
C8H8(g)+H2(g)��H=120kJmol-1ij�¶��£���0.40mol�����飬����2L����ܱ������з�����Ӧ���ⶨ��ͬʱ����������������ʵ������õ��������±���
��1������Ӧ���е�20minʱ���ö�ʱ����H2��ƽ����Ӧ������__��
��2�����¶��£���Ӧ��ƽ�ⳣ������ʽ��K=____��K��ֵ��__��
��3�������������������䣬��0.50molH2(g)��0.50molC8H8(g)�ϳ�C8H10(g)������30kJ�����ų�ʱ���÷�Ӧ��H2��ת������__����ʱ���úϳɷ�Ӧ�Ƿ�ﵽ��ƽ��״̬��__����������������������������Ӧ����__�淴Ӧ���ʣ�����ڡ�С�ڻ���ڣ���
���𰸡�0.004mol��L-1��min-1 ![]() 0.1 50% �� ����
0.1 50% �� ����
��������
����ͼ�����ݼ����ʱ���ʽ�����������ʣ����������Ϣ��ƽ��ʱ���ʵ�Ũ�ȼ���ƽ�ⳣ����ת���ʣ�������ƽ�ⳣ���жϷ�Ӧ�Ƿ�ﵽƽ�⼰���淴Ӧ���ʴ�С��
��1����0.40mol�����飬����2L����ܱ������з�����Ӧ��20min��ʣ��0.24mol��������0.16mol����

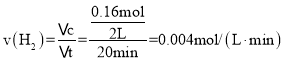 ���ʴ�Ϊ��0.004mol��L-1��min-1��
���ʴ�Ϊ��0.004mol��L-1��min-1��
��2�����������Ϣ֪��ƽ��ʱ������ϩ(C8H8)Ϊ0.2mol����

��ѧƽ�ⳣ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() �� 0.1��
�� 0.1��
��3����C8H10(g) ![]() C8H8(g)+H2(g) ��H=120kJmol-1����֪C8H8(g)+H2(g)
C8H8(g)+H2(g) ��H=120kJmol-1����֪C8H8(g)+H2(g) ![]() C8H10(g) ��H=120kJ��mol1����30kJ�����ų�ʱ�����ĵ�H2��Ϊ0.25mol����������ת����=
C8H10(g) ��H=120kJ��mol1����30kJ�����ų�ʱ�����ĵ�H2��Ϊ0.25mol����������ת����=![]() �����ݣ�2������ƽ�ⳣ��=0.1��֪C8H8(g)+H2(g)
�����ݣ�2������ƽ�ⳣ��=0.1��֪C8H8(g)+H2(g) ![]() C8H10(g)��ƽ�ⳣ��Ϊ
C8H10(g)��ƽ�ⳣ��Ϊ![]() ����
����

![]() ��8<10����Ӧδ��ƽ��״̬�����������ƶ�������Ӧ���ʴ����淴Ӧ���ʣ��ʴ�Ϊ��50%�����ڡ�
��8<10����Ӧδ��ƽ��״̬�����������ƶ�������Ӧ���ʴ����淴Ӧ���ʣ��ʴ�Ϊ��50%�����ڡ�