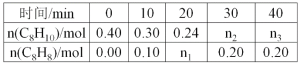
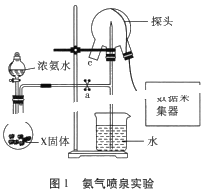
��Ŀ����
����Ŀ����ҵ��ˮ�г�����һ������Cr2O72-��CrO42-�����ǻ�����༰��̬ϵͳ�����ܴ�Σ����������д��������õĴ����������������֣�
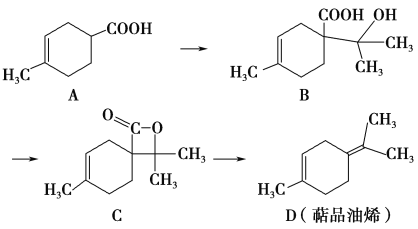
����1����ԭ������
�÷��Ĺ�������Ϊ��
CrO42-![]() Cr2O72-
Cr2O72-![]() Cr3+
Cr3+![]() Cr(OH)3
Cr(OH)3
���еڢٲ�����ƽ��2CrO42-����ɫ��+2H+![]() Cr2O32-����ɫ��+H2O
Cr2O32-����ɫ��+H2O
(1)��ƽ����ϵ��pH=2������Һ��______ɫ��
(2)��˵���ڢٲ���Ӧ��ƽ��״̬����_____������ţ�
A��Cr2O72-��CrO42-��Ũ����ͬ
B��2v��Cr2O72-��=v��CrO42-��
C����Һ����ɫ����
(3)�ڢڲ��У���ԭ1mol Cr2O72-���ӣ���Ҫ______mol��FeSO4��7H2O��
(4)�ڢ۲����ɵ�Cr(OH)3����Һ�д������³����ܽ�ƽ�⣺Cr(OH)3(s) ![]() Cr3+(aq)+3OH(aq)�������£�Cr(OH)3���ܶȻ�Ksp=c(Cr3+)��c3(OH)=1032��Ҫʹc(Cr3+)����105mol/L����Һ��pHӦ����______��
Cr3+(aq)+3OH(aq)�������£�Cr(OH)3���ܶȻ�Ksp=c(Cr3+)��c3(OH)=1032��Ҫʹc(Cr3+)����105mol/L����Һ��pHӦ����______��
����2����ⷨ
�÷���Fe���缫��⺬Cr2O72-�����Է�ˮ�����ŵ��Ľ��У�������������ҺpH���ߣ�����Cr(OH)3������
(5)��Fe���缫��ԭ��Ϊ______���õ缫��Ӧʽ���ͣ���
(6)������������ҺpH���ߣ���Һ��ͬʱ���ɵij�������______��
���𰸡��� C 6 5 ������ӦΪFe2e=Fe2+���ṩ��ԭ��Fe2+ Fe(OH)3
��������
(1)��Һ�����ԣ�c(H+)�ϴ�����ƽ�����ƣ�����Һ�Գ�ɫ��
(2)A����Ӧ�����п��ܳ���Cr2O72-��CrO42-��Ũ����ͬ������Ӧ��һ���ﵽƽ��״̬��A����
B��2v��Cr2O72-��=v��CrO42-����û�б�����淴Ӧ���ʣ����ж���Ӧ�Ƿ�ﵽƽ��״̬��B����
C��ƽ��ʱ�����ʵ�Ũ�Ȳ��ٸı䣬����Һ����ɫ���ٸı䣬�����жϷ�Ӧ�ﵽƽ��״̬��C��ȷ����������ѡC��
(3)���ݵ��ӵ�ʧ�غ����֪������ԭ1mol Cr2O72-���ӵõ�Cr3+���õ����ӣ�2����6-3��=6mol��Fe2+������ΪFe3+����ҪFeSO4��7H2O�����ʵ���Ϊ![]() =6mol��
=6mol��
(4)��c(Cr3+)=105mol/Lʱ����Һ��c(OH)=![]() mol/L =109mol/L��c(H+)=
mol/L =109mol/L��c(H+)=![]() mol/L =105mol/L��pH=5����Ҫʹc(Cr3+)����105mol/L����Һ��pHӦ����5��
mol/L =105mol/L��pH=5����Ҫʹc(Cr3+)����105mol/L����Һ��pHӦ����5��
(5)��Fe������������������Ӧ��ʧ���ӣ�Fe2e=Fe2+��������������������ԭ����Cr2O72-��ԭ��Cr3+��
(6)��Һ���������������õ��ӱ���ԭΪ��������������ӦΪ��2H++2e=H2������Һ���Լ�������ҺpH���ߣ��������ӱ�����Ϊ�����ӣ����Լ����������Ӳ�������Fe(OH)3��