��Ŀ����
16������������ȷ���ǣ�������| A�� | S��g��+O2��g���TSO2��g����H=a��S��s��+O2��g���TSO2��g����H=b����a��b | |
| B�� | C��ʯī��s���TC�����ʯ��s����H=+1.9 kJ/mol������ж�ʯī�Ƚ��ʯ�ȶ� | |
| C�� | NaOH��aq��+HCl��aq���TNaCl��aq��+H2O��l����H=-57.4 kJ/mol����20 g NaOH����Һ��ϡ������ȫ��Ӧ���ų�������Ϊ28.7 kJ | |
| D�� | CaCO3��s���TCaO��s��+CO2��g����H��0����÷�Ӧ�κ��¶��¶����Է����� |
���� A����̬S�ȹ�̬S���������ߣ����ȡ�H��0��
B�����ʵ�����Խ��Խ���ȶ���
C������NaOH�����ʵ������к��ȼ��㣻
D����Ӧ�ڸ��������²��ܽ��У�
��� �⣺A�����ȡ�H��0����̬S�ȹ�̬S���������ߣ�������̬S��Ӧ���ȶ࣬����Խ���ʱ�ԽС������a��b����A����
B�����Ȼ�ѧ����ʽ��֪��ʯī������С�ڽ��ʯ����ʯī���ȶ�����B��ȷ��
C��20 g NaOH��n��NaOH��=0.5mol���ų�������Ϊ0.5mol��57.4kJ/mol=28.7kJ����C��ȷ��
D��CaCO3��s��=CaO��s��+CO2��g����H��0����S��0���ɡ�G=��H-T•��S��֪�����ڽϸ��¶��·�Ӧ�����Է����У���D����
��ѡBC��
���� ���⿼�鷴Ӧ�ȼ��ʱ䣬��Ŀ�Ѷ��еȣ�����ע������Ȼ�ѧ����ʽ�����壬���ձȽϷ�Ӧ�ȵķ�����

��ϰ��ϵ�д�
�����Ŀ
5�����淴ӦN2+3H2?2NH3�������淴Ӧ���ʿ��ø���Ӧ���������Ũ�ȵı仯����ʾ�����и���ϵ����˵����Ӧ�Ѵﵽƽ��״̬���ǣ�������
| A�� | 3������N2��=������H2�� | B�� | ������N2��=������NH3�� | C�� | ������N2��=3������H2�� | D�� | 2������H2��=3������NH3�� |
7��һ���¶�ʱ����2.0L�����ܱ������г���1��omolPCl5��������Ӧ��
PCl5��g��?PCl3��g��+Cl2��g��
��һ��ʱ���Ӧ�ﵽƽ�⣬��Ӧ�����в�õIJ������ݼ��±���
����˵����ȷ���ǣ�������
PCl5��g��?PCl3��g��+Cl2��g��
��һ��ʱ���Ӧ�ﵽƽ�⣬��Ӧ�����в�õIJ������ݼ��±���
| ��Ӧʱ��/s | 0 | 50 | 150 | 250 | 350 |
| n��PCl3��/mol | 0 | 0.16 | 0.19 | 0.20 | 0.20 |
| A�� | ��Ӧ��ǰ50s��ƽ������v��PCl5��=0.0032mol•L-1•s-1 | |
| B�� | ���������������䣬�������¶ȣ���Ӧ���´ﵽƽ�⣬ƽ��ʱc��PCl3��=0.11mol•L-1��������Ӧ�ġ�H��0 | |
| C�� | ��ͬ�¶��£�����ʼʱ�������г���1.0molPCl5��0.20��molCl2���ڷ�Ӧ�ﵽƽ��ǰv��������v���棩 | |
| D�� | ��ͬ�¶��£�����ʼʱ�������г���1.0molPCl5��1.0��molCl2���ڷ�Ӧ�ﵽƽ��ʱPCl5��ת����Ϊ80% |
11����һ�������£�����ҩ�����Ҫ�ɷֶ��ܷ�����ȡ����Ӧ �ڼӳɷ�Ӧ ��ˮ�ⷴӦ ���кͷ�Ӧ���ַ�Ӧ���ǣ�������
| A�� | 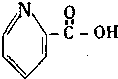 ά���� | B�� | 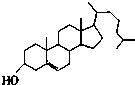 ���̴� | ||
| C�� | 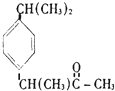 �ұص� | D�� | 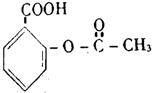 ��˹ƥ�� |
5��ʯīϩ���ɵ���̼ԭ�ӹ��ɵ����Ͳ��ϣ�����̫���ܵ�صĵ缫�����й���ʯīϩ�ķ��࣬��ȷ���ǣ�������
| A�� | ���ڹ��ۻ����� | B�� | �����л��� | C�� | ���ڵ��� | D�� | ���ڵ���� |
6�������ͺͲ�����Ȼ�����������͡������ӻ�ѧ��ɺͷ��ӽṹ��������������ȫ��ͬ�ģ�����˵������ȷ���ǣ�������
| A�� | ���������ڴ�����������ڻ���� | |
| B�� | �������������࣬������������ | |
| C�� | ������������������������ | |
| D�� | ���������ڸ߷��ӻ������������С���ӻ����� |

