��Ŀ����
���в��ֶ���������Ԫ�ص��й���Ϣ�����±���
��1��Y�����������Ų�ʽ��__ __�����ĵ�����̼���ɵĻ�������ˮ��Ӧ���ɼ���Ͱ�ɫ������д���÷�Ӧ�Ļ�ѧ����ʽ__ ��
��2��Ԫ��T�ĵ�����ˮ��Ӧ�����ӷ���ʽ�� ��
�ڶ���������Ԫ���У�XԪ����������Ԫ�ص�ԭ�Ӱ뾶��С�����˳����_
��дԪ�ط��ţ���
��3��W�γɵ�һ�ֵ��ʣ���ʽ��Ϊ256��������CS2���õ��ʵĻ�ѧʽΪ___ __��������_ ___���壨д�������ͣ���
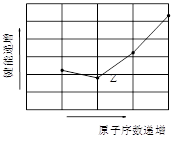
��4����ͼΪZԪ������������̬�⻯��R-H���ļ��ܴ�С���������Ԫ����̬�⻯����ܴ�С������Ĺ�ϵΪ_____ �������ּ�������
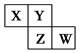
| Ԫ�ر�� | T | X | Y | Z | W |
| Ԫ�ص����ʻ�ԭ�ӽṹ��� | ����������Ԫ����ԭ�Ӱ뾶��� | ������ϵĵ������ȴ������1���ҵ��������� | �����13���˶�״̬��ͬ�ĵ��� | ����������Һ̬����������е�ϵͿ��Ȼ�����ĵ��� | ������5�ֲ�ͬ�����ĵ���������������δ�ɶԵĵ��� |
��1��Y�����������Ų�ʽ��__ __�����ĵ�����̼���ɵĻ�������ˮ��Ӧ���ɼ���Ͱ�ɫ������д���÷�Ӧ�Ļ�ѧ����ʽ__ ��
��2��Ԫ��T�ĵ�����ˮ��Ӧ�����ӷ���ʽ�� ��
�ڶ���������Ԫ���У�XԪ����������Ԫ�ص�ԭ�Ӱ뾶��С�����˳����_
��дԪ�ط��ţ���
��3��W�γɵ�һ�ֵ��ʣ���ʽ��Ϊ256��������CS2���õ��ʵĻ�ѧʽΪ___ __��������_ ___���壨д�������ͣ���
��4����ͼΪZԪ������������̬�⻯��R-H���ļ��ܴ�С���������Ԫ����̬�⻯����ܴ�С������Ĺ�ϵΪ_____ �������ּ�������

��1��3s23p1��1�֣��� Al4C3 + 12H2O ��4Al(OH)3 +3CH4����1�֣���
��2��2Na + 2H2O��2Na+ +2OH- +H2������1�֣��� F��Cl��S��1�֣���
��3��S8����1�֣� ���ӣ�1�֣���
��4����CH4�⣬������С����������2�֣�����CH4�⡱��дֻ��1�֣�
��2��2Na + 2H2O��2Na+ +2OH- +H2������1�֣��� F��Cl��S��1�֣���
��3��S8����1�֣� ���ӣ�1�֣���
��4����CH4�⣬������С����������2�֣�����CH4�⡱��дֻ��1�֣�
�����������������������Ϣ��֪��T��Na��X��Cl��Y��Al ��Z��N��W��Si ��
��1��Al �����������Ų�ʽΪ��3s23p1�����ĵ�����̼���ɵĻ�������ˮ��Ӧ��Al4C3 + 12H2O ��4Al(OH)3 +3CH4����
��2���Ƶĵ�����ˮ��Ӧ�����ӷ���ʽ�ǣ�2Na + 2H2O��2Na+ +2OH- +H2����ͬ���ڴ������Ұ뾶��С֪�� F��Cl��S��
��3������ʽ��֪��S8�� ������CS2��֪Ϊ���Ӿ��壻
��4�����ͼ���֪����CH4�⣬������С����������

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 ���Ӻ�������Ľ�Ϊ�˽���ʵ�����������ԭ�ӵĺ�ʽ�ṹѧ˵������ͼ�У��ڵ��ʾ��ԭ�Ӻ˵�λ�ã�����ab��cd��ef��ʾ������ԭ�Ӻ˸�����
���Ӻ�������Ľ�Ϊ�˽���ʵ�����������ԭ�ӵĺ�ʽ�ṹѧ˵������ͼ�У��ڵ��ʾ��ԭ�Ӻ˵�λ�ã�����ab��cd��ef��ʾ������ԭ�Ӻ˸�����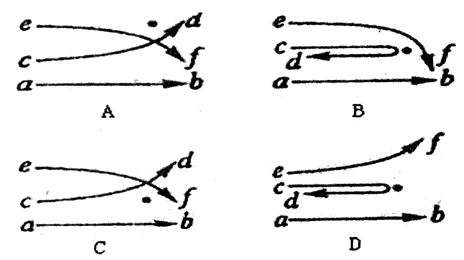
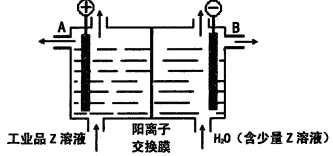
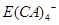 ����ʽ���ڣ�д��E����Z��Һ�����ӷ���ʽ��_________________________________________.
����ʽ���ڣ�д��E����Z��Һ�����ӷ���ʽ��_________________________________________.