��Ŀ����
��ʽ̼��ͭ��һ�ֻ���ԭ�ϣ���ѧʽ��mCu(OH)2��nCuCO3��ʾ��ʵ�����Է�ͭмΪԭ����ȡ��ʽ̼��ͭ�IJ������£�
��.��ͭм������ͭ
����1����ͭм�ڿ����г�����գ�������������ϡ���
����2����ͼ1(�г�������ʡ��)����Ũ���Ỻ���ӵ���ͭм��(��ͭм����)����ַ�Ӧ����ˣ��õ�����ͭ��Һ��
����3��������2��Ũ���ỻ��ϡ���ᣬ�������䡣
��.��ʽ̼��ͭ���Ʊ�
������Թ��м���̼������Һ������ͭ��Һ
��ˮԡ������70 ������
����0.4 mol��L��1��NaOH��Һ����pH��8.5�������á�����
������ˮϴ�ӡ���ɣ��õ���ʽ̼��ͭ��Ʒ
��ش��������⣺
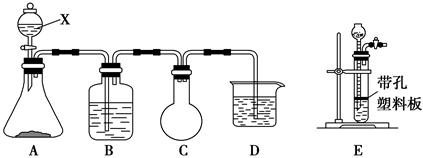
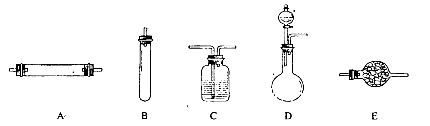
(1)������1ʵ�飬����ѡ�������������________(�����)��
(2)ͼ2���ֱܷ����ͼ1��B��Cװ�õ���________(��װ�����)��
(3)��֪��NO��NO2��2NaOH===2NaNO2��H2O��2NO2��2NaOH===NaNO3��NaNO2��H2O��NO���ܵ�����NaOH��Һ��Ӧ��ʵ�����ʱ����β�������ʹװ���е��ж����屻NaOH��Һ��ȫ���գ�__________________________��
(4)�������ϴ�ӵ�Ŀ����______________________________________��
(5)����۹��˺����Һ�к���CO32��������CO32���ķ�����_________________________________________________________��
(6)�ⶨ��ʽ̼��ͭ��ɵķ�����Ҫ�����֣�
����1�����շ���ȡ34.6 g������mCu(OH)2��nCuCO3����Ӳ���Թ������գ��������������ͨ��������Ũ���ᡢ�����ļ�ʯ���У���ȫ���պ�Ũ���Ά��1.8 g����ʯ�Ҿ���8.8 g��
����2����ԭ�����������м�ǿ�ȣ��ⷴӦǰ������������
�������������������ʽ̼��ͭ�Ļ�ѧʽ_____________________��
����ƽ��ѧ����ʽ��mCu(OH)2��nCuCO3��________H2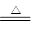 ________Cu��________CO2����________H2O
________Cu��________CO2����________H2O
��(1)abe��(2)��͢�(3)�رջ���b������a��ͨ��һ��ʱ�������(4)��ȥ��Ʒ�������ʡ�(5)ȡ������Һ���Թ��У�����ϡ���ᣬ������������ͨ�����ʯ��ˮ�У���Һ����ǣ�˵����Һ�к���CO32��
(6)��Cu(OH)2��2CuCO3����(m��n)��(m��n)
n��(2m��n)
����

��ˮ�Ȼ���������������ϵ������ӡȾ��ýȾ����Ⱦ�ϻ�ԭ������������ұ��ҽҩ���������ҵ��һʵ��С��ģ�¹�ҵ������ȡ�Ȼ����������װ������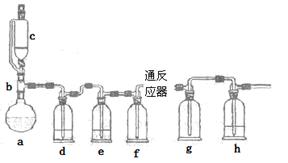
ͨ������������Ͽ�֪��
���ڳ�������500��ʱ�����봿��������Cl2��Ӧ������FeCl2�����¶Ƚϵ�ʱ������FeCl3��
��FeCl3�۷е�ͣ���������
����������Ϣ�ش���ص�����
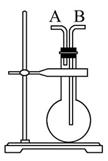
��1��abc������ϳ���ȡ������װ�ã���Ӧ������ȣ���д��a�������������Ļ�ѧ��Ӧ����ʽ ��
��2��d��eʢװ��ҩƷ�ֱ��� �� ��f��g�������� ��
��3����Ӧ��ΪӲ�ʲ����ܣ�����������������500�����ҷ�Ӧ��
�ٻ�ѧ��Ӧ����ʽΪ
��ʵ�����˳���ǣ���װ������ ��װ��ҩƷ�� �� ��ֹͣ���ȡ��ر�c�Ļ�����
��4��ʵ��С���¼��ʵ���������£�
| | �۲쵽�IJ������� |
| ��һ��ʵ�� | ��Ӧ�����а�������ɫ���塢gƿ�а����ͻ���ɫ���� |
| �ڶ���ʵ�� | ��Ӧ��������ɫ���壬gƿ�к���ɫ���̺ͻ���ɫ���� |
| ������ʵ�� | ��Ӧ��������ɫ���壬gƿ�л���ɫ���� |
�ٵ�һ��ʵ�飬����eƿû��ʢװ�κ�ҩƷ�����Եõ���ɫ���壬��ԭ���� ��
�ڵڶ���ʵ�飬gƿ�к���ɫ���̣���ԭ���� ��
��5��������ʵ��õ��Ĺ��壬����ܺ����������� �������Ҫ����Լ2��3mol/L��Ⱦ�ϻ�ԭ����Һ���������ȥ�������� ��

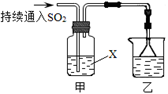
 Ca2��+
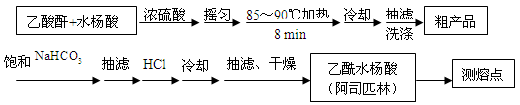
Ca2��+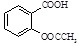 ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135 �档ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ��Ļ��������������£�
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135 �档ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ��Ļ��������������£�