题目内容
1. 乙醇是一种重要的有机化工原料,它可以用玉米、薯类等为原料经发酵、蒸馏制成.乙醇和汽油经加工处理形成的车用燃料即乙醇汽油.结合有关知识,回答下列问题:
乙醇是一种重要的有机化工原料,它可以用玉米、薯类等为原料经发酵、蒸馏制成.乙醇和汽油经加工处理形成的车用燃料即乙醇汽油.结合有关知识,回答下列问题:(1)乙醇分子中的官能团是羟基(-OH)
(2)在试管里加入2mL乙醇.把一端弯成螺旋状的铜丝放在酒精外焰中加热,使铜丝表面生成一薄层黑色的氧化铜,立即把它插入盛有乙醇的试管里(如图),取出铜丝,可以观察到铜丝表面铜丝变红色.
(3)乙醇汽油作为一种新型车用燃料,符合我国的能源战略,推广使用乙醇汽油的好处是(写出一条即可)乙醇燃烧的热值高,乙醇是可再生能源,可以减少汽油的使用量.
分析 (1)醇的官能团为羟基;
(2)乙醇能与黑色的氧化铜反应得到乙醛和红色的铜以及水;
(3)乙醇优点很多,如:环保、节省能源、新的清洁型能源等.
解答 解:(1)乙醇结构简式为:CH3CH2OH,分子中的官能团是羟基(-OH),
故答案为:羟基(-OH);
(2)金属铜在乙醇氧化生成乙醛的反应中作催化剂,螺旋状的铜丝放在酒精灯外焰部分加热,铜被氧气氧化为黑色氧化铜,氧化铜能将乙醇氧化为乙醛,自身被还原成铜,反应方程式为2C2H5OH+O2→Cu△→Cu△2CH3CHO+2H2O,所以观察到铜丝表面变红色,
故答案为:铜丝变红色;
(3)乙醇燃烧的热值高,乙醇完全燃烧产物为二氧化碳和水,对环境无污染,属于可再生能源,使用乙醇汽油节可约化石燃料,
故答案为:乙醇燃烧的热值高,乙醇是可再生能源,可以减少汽油的使用量.
点评 本题考查了乙醇的化学性质,注意乙醇催化氧化的原理、氧化还原反应等知识,题目难度不大.

练习册系列答案
相关题目
11.NA为阿伏加德罗常数的值.下列叙述正确的是( )
| A. | 标准状况下,22.4LC2H2含σ键3NA,π键2NA | |
| B. | 在含4molSi-O键的二氧化硅中,氧原子数为4NA | |
| C. | 1.8gH218O中含有的中子数为NA | |
| D. | 标准状况下,7.1g氯气与足量石灰乳充分反应转移电子数为0.1NA |
12.为证明溴乙烷在NaOH的乙醇溶液中加热发生消去反应,可将反应后的气体通入( )
| A. | 溴水 | B. | AgNO3溶液 | C. | 酸性KMnO4溶液 | D. | 酸化的AgNO3溶液 |
9.下列物质的说法不正确的是( )
| A. | 液化石油气是通过石油催化裂化或裂解得到的 | |
| B. | 芳香烃主要来自于煤的干馏后的煤焦油 | |
| C. | 汽油、煤油、柴油主要来自于石油的常压蒸馏 | |
| D. | 乙烯是石油裂解后的产物 |
16.家里的食用花生油中如果混有水份,可以采用下列何种方法分离( )
| A. | 过滤 | B. | 蒸馏 | C. | 分液 | D. | 结晶 |
6.同周期的ⅡA族元素与ⅢA族元素的原子序数之差的绝对值( )
| A. | 一定1 | B. | 一定是11 | C. | 一定是1或11 | D. | 以上均不正确 |
13.为检验Na2SO3溶液中是否混有Na2SO4,应使用的试剂是( )
| A. | BaCl2溶液 | B. | Ba(NO3)2溶液 | ||
| C. | BaCl2溶液和稀H2SO4 | D. | BaCl2溶液和稀盐酸 |
10.关于下列各装置图的叙述中,正确的是( )
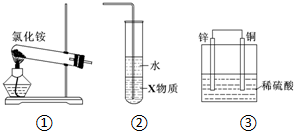

| A. | 实验室用装置①制取氨气 | |
| B. | 装置②中X若为四氯化碳,可用于吸收氨气,并防止倒吸 | |
| C. | 装置③是电解池,Cu上产生气泡 | |
| D. | 装置③是原电池,锌电极为阴极,发生氧化反应 |