��Ŀ����
����˵����ȷ����
�ٱ�״���£�22.4 L��ϩ���еķ�����Ϊ6.02��1023
�ڱ�״���£�a L�������͵����Ļ���ﺬ�еķ�����ԼΪ ��6.02��1023
��6.02��1023
��7.1 g����������������������Һ��Ӧת�Ƶĵ�����ԼΪ0.2��6.02��1023
��1 mol�Ҵ��к��еĹ��ۼ���ԼΪ7��6.02��1023
��500 mL 1 mol/L����������Һ�к��е������������ԼΪ1.5��6.02��1023
| A���٢� | B���ڢ� | C���ۢ� | D���ڢ� |
B
��������������� ��ϩ�ڱ�״����ΪҺ�壬����Ħ��������㣻�� �����͵����ڱ�״���£����Ը���Ħ��������㣬��ȷ���� 7.1 g����������������������Һ��Ӧת�Ƶĵ�����ԼΪ0.1��6.02��1023������ 1 mol�Ҵ��к��еĹ��ۼ���ԼΪ8��6.02��1023������ �������������0.5L��1 mol��3��6.02��1023 mol��1����ȷ����ѡB��
���㣺���⿼���й����ʵ����ļ��㡣

��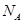 ��ʾ�����ӵ�������ֵ������������ȷ����
��ʾ�����ӵ�������ֵ������������ȷ����
A��1.7gH2O2�к��е�������ĿΪ0.9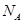 |
B��1molNa2O2�����к���������Ϊ4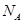 |
C����״���£�0.1molCl2����ˮ��ת�Ƶ�����ĿΪ0.1 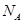 |
D��25�棬pH=13��NaOH��Һ�к���OH������ĿΪ0.1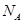 |
�о����֣�����Խϡ����ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡ�����������Ͻ���������ϡ�������ַ�Ӧ��û������ų����ڷ�Ӧ���������Һ�У���μ���5 mol��L��NaOH��Һ������NaOH��Һ�������mL��������ij��������ʵ�����mol���Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
| A����Һ�н��OH��������ǿ��������NH4�� |
| B��D��ʱ��Һ�д��ڣ�c(NH4��)��c(H��)��c(OH��)��c(NO3��) |
| C���Ͻ���n(Fe) ��n(Al)��1 ��3 |
| D��C��ֵΪ7 |
��NA��ʾ�����ӵ�������ֵ������������ȷ����
| A��0.1 mol Fe��0.1mol CL2�г��ȼ�գ�ת�Ƶĵ�����Ϊ0.3NA |
| B����״���£�11.2 L CCl4����������ԼΪ0.5NA |
| C�������£�pH=13��NaOH��Һ�У�OH������ĿΪ0.1NA |
| D�����¡���ѹ�£�14g CO��N2�Ļ��������ԭ����ΪNA |
��Mg��Cu��ɵĻ����26.4gͶ�뵽������ϡ�����У�������ȫ�ܽ⣬�ռ�����״���µ�NO����8.96L����Ӧ�����Һ�м��������5mol��L-1��NaOH��Һ300mL������������ȫ���������γɳ����������� ( )
| A��43.2g | B��46.8g | C��53.6g | D��63.8g |
��״����, 1���ˮ����560����������ð�ˮ���ܶ���0.91g / mL, �ð�ˮ�����ʵ���Ũ��Լ��
| A��4 mol / L | B��8 mol / L | C��10 mol /L | D��16 mol / L |
�о����֣�����Խϡ����ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡ�����������Ͻ���������ϡ�������ַ�Ӧ��û������ų����ڷ�Ӧ���������Һ�У���μ���4mol/LNaOH��Һ������NaOH��Һ�����(mL)������ij��������ʵ���(mol)�Ĺ�ϵ��ͼ��ʾ��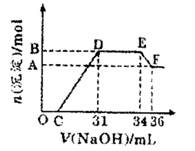
��������ͼ�Σ�����˵������ȷ����
| A��OC�����ӷ�Ӧ����ʽ��H+ʮOH-=H2O |
| B����Һ�н��OH-������ǿ��������H+��������������Al3+ |
| C����Һ��n(NH4+)=0.012mol |
| D�����ⶨF�������������ʵ�鲽���ǣ����ˡ�ϴ�ӡ�������� |
þ����ʽ�ζԹ�ҵ��ˮ�еĸ������������ԡ�ȡ�ü�ʽ��0.7525g���μ�1.0mol/L���ᣬ��������21.25mLʱ��ʼ����CO2���壬����������22.50mLʱǡ�÷�Ӧ��ȫ�������Һ�м����������������Һ�����ˣ���������и������0.4350g����ü�ʽ�εĻ�ѧʽ��
| A��Al2Mg6(OH)16CO3?4H2O | B��Al4Mg12(OH)34CO3?8H2O |
| C��Al2Mg4(OH)16CO3?4H2O | D��Al3Mg6(OH)34CO3?8H2O |
NA��ʾ�����ӵ��������й�NA����ȷ˵����
| A��1mol D318O+�к��е�������Ϊ10NA |
| B����״���£�22.4Lˮ�к�O��H����Ϊ2NA |
| C��ij�¶�ʱ1L pH��6�Ĵ�ˮ����OH��������Ϊ1.0��10��6NA |
| D��7.8 g Na2S��Na2O2�Ļ�����к��е�������������0.1 NA |