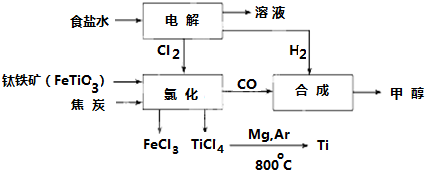
��Ŀ����
13������ҪŨ��Ϊ0.5000mol/L��ϡ����480mL��ʵ��Ա����98%��Ũ�������ƣ���1��ѡ�õ���Ҫ�����У�
����Ͳ�����ձ����۲���������500mL����ƿ���ݽ�ͷ�ιܣ�
��2����Ҫ�ش��������⣺
������Ũ��������ʵ���Ũ��Ϊ18.4 mol/L��
������Ũ��������Ϊ13.6mL��
�����ϡ��Ũ���ὫŨ�������ձ��ڱ�����ע��ʢˮ���ձ��У����ò��������Ͻ��裮
��3�������ƹ����У���������������ȷ�ģ����в���������������ҺŨ��ƫ�ߵ��Ǣڢ�
������Ͳ��ȡŨ����ʱ�����Ӷ���
��δ��ȴ�����¾�ת����Һ�����ݣ�
�۶���ʱ���ӿ̶��ߣ�
�ܶ��ݺ�����ƿ������ҡ�ȣ����ú���Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�
���� ��1����������һ�����ʵ���Ũ����Һ��һ�㲽��ѡ��ʹ��������
��2��������C=$\frac{1000�Ѧ�}{M}$����Ũ��������ʵ���Ũ�ȣ�
���ٸ���ϡ�Ͷ��ɼ�������Ũ����������
������Ũ����ϡ�͵���ȷ�������
��3���������������ʵ����ʵ�������Һ�������Ӱ�죬����c=$\frac{n}{V}$�ж϶�Ũ�ȵ�Ӱ�죮
��� �⣺��1������һ�����ʵ���Ũ����Һ��һ�㲽�裺���㡢��ȡ��ϡ�͡���Һ��ϴ����Һ�����ݡ�ҡ�ȵȲ������õ��������У���Ͳ���ձ�����������500mL����ƿ ��ͷ�ιܣ����Ի�ȱ�ٵ�����Ϊ��500mL����ƿ ��ͷ�ιܣ�
�ʴ�Ϊ��500mL����ƿ ��ͷ�ιܣ�
��2����Ũ������ܶ�C=$\frac{1000��1.84��98%}{98}$=18.4mol/L��
�ʴ�Ϊ��18.4��
������Ũ��������ΪV������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬����V��18.4mol/L=500mL��0.5mol/L����ã�V=13.6mL����Ũ��������Ϊ13.6mL��
�ʴ�Ϊ��13.6��
��Ũ����ϡ�͵���ȷ��������Ϊ����Ũ�������ձ��ڱ�����ע��ʢˮ���ձ��У����ò��������Ͻ��裻
�ʴ�Ϊ����Ũ�������ձ��ڱ�����ע��ʢˮ���ձ��У����ò��������Ͻ��裻
��3��������Ͳ��ȡŨ����ʱ�����Ӷ�����������ȡ��Ũ�������ƫС����������ʵ���ƫС����ҺŨ��ƫ�ͣ��ʲ�ѡ��
��δ��ȴ�����¾�ת����Һ�����ݣ���ȴ��Һ���½�����Һ���ƫС����ҺŨ��ƫ�ߣ���ѡ��
�۶���ʱ���ӿ̶��ߣ�������Һ���ƫС����ҺŨ��ƫ�ߣ���ѡ��
�ܶ��ݺ�����ƿ������ҡ�ȣ����ú���Һ����ڿ̶��ߣ��ټ�ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ��ʲ�ѡ��
��ѡ���ڢۣ�
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ���Ͳ��������ǽ���ؼ���ע���������ķ����ͼ��ɣ���Ŀ�ѶȲ���

| A�� | C60��Ħ��������720 | |
| B�� | ���ʯ��ʯī��C60��Ϊͬλ�� | |
| C�� | ��ʯī���е����ԣ�����ʯīը���ƻ�����ߡ��糧�豸 | |
| D�� | �ڵ�ȼ�������£�̼���Ի�ԭ����þ����þ�Ͷ�����̼ |

��֪���ٻ���������Cl2��Ӧ����PCl3�������Cl2��Ӧ����PCl5��
��PCl3��ˮ��ǿ��ˮ������ H3PO3�����壻
��PCl3��O2������POCl3��POCl3����PCl3��
��PCl3��POCl3���۷е���±���
| ���� | �۵�/�� | �е�/�� |
| PCl3 | -112 | 75.5 |
| POCl3 | 2 | 105.3 |
��1��B����װ�Լ���Ũ���
E����ˮ������������PCl3��ֹ��ӷ���
��2��F�м�ʯ�ҵ����������ն������������ֹ�����е�ˮ����������ƿ�к�PCl3 ��Ӧ��
��3��ʵ��ʱ�����װ�������Ժ��ȴ�K3ͨ������CO2����Ѹ�ټ�����ף�ͨ����CO2���������ž�װ���еĿ�������ֹ������ȼ��
��4���ֲ�Ʒ�г�����POC13��PCl5�ȣ���������ȳ�ȥPCl5��ͨ��������ʵ��������ƣ������ɵõ��ϴ�����PCl3��
��5��ʵ�����ʱ����������C�е��Լ����ն����������C�з�Ӧ�����ӷ���ʽΪCl2+2OH-=Cl-+ClO-+2H2O��
��6��ͨ�����淽���ɲⶨ��Ʒ��PCl3������������
��Ѹ�ٳ�ȡ1.00g��Ʒ����ˮ��Ӧ�����250mL��Һ��
��ȡ������Һ25.00mL�������м���10.00mL 0.1000mol/L��ˮ����ַ�Ӧ��
�����������Һ�м��뼸�ε�����Һ����0.1000mol/L��Na2S2O3����Һ�ζ���
���ظ��ڡ��۲�����ƽ������Na2S2O3��Һ8.40mL��
��֪��H3PO3+I2=H3PO4+2HI��I2+2Na2S2O3�T2NaI+Na2S4O6�������������ݣ�����ⶨ������û��������Ӧ���ò�Ʒ��PCl3����������Ϊ79.75%��
| A�� | 2NH3+3CuO��3Cu+N2+3H2O | B�� | 2Na+2NH3��2NaNH2+H2�� | ||
| C�� | 4NH3+6NO��5N2+6H2O | D�� | 3SiH4+4NH3��Si3N4+12H2 |
| A�� | SO2����ɫʯ����ֽ�ȱ�����ɫ�� | B�� | ��ˮ��pH��ֽ�ȱ�����ɫ�� | ||
| C�� | Na2S��pH��ֽ��죩 | D�� | KI��������ֽ������ |
��֪��

����˵����ȷ���ǣ�������
| A�� | H2��I2��HI�����еĻ�ѧ�����ǷǼ��Թ��ۼ� | |
| B�� | �Ͽ�2 mol HI�����еĻ�ѧ����������ԼΪ��c+b+a��kJ | |
| C�� | ��ͬ�����£�1molH2��g����1mol I2��g��������С��2mol HI��g���������� | |
| D�� | ���ܱ������м���2mol H2��g����2mol I2��g������ַ�Ӧ��ų�������Ϊ2akJ |
| A�� | ƽ���ƶ���Kֵһ���仯 | B�� | Kֵ�仯��ƽ��һ���ƶ� | ||
| C�� | ƽ���ƶ���Kֵ���ܲ��� | D�� | Kֵ���䣬ƽ������ƶ� |
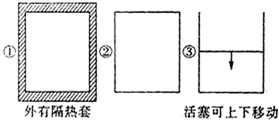 ����ͼ��ʾ���ݻ���ͬ�����������٢ڢ��н������·�Ӧ��3A��g��+B��g��?2C��g����H��0������ʼ�¶���ͬ���ֱ������������г���6mol A��2mol B����ﵽƽ��ʱ��������C���ʵİٷֺ����ɴ�С��˳��Ϊ�ۢڢ٣���������ţ���
����ͼ��ʾ���ݻ���ͬ�����������٢ڢ��н������·�Ӧ��3A��g��+B��g��?2C��g����H��0������ʼ�¶���ͬ���ֱ������������г���6mol A��2mol B����ﵽƽ��ʱ��������C���ʵİٷֺ����ɴ�С��˳��Ϊ�ۢڢ٣���������ţ���