��Ŀ����
�ס��ҡ�?������Ϊǰ������Ԫ���γɵ����ӣ����ǵĵ���������ȡ���֪�ס��ҡ���Ϊ˫ԭ�ӷ��ӻ���˫ԭ�������ӣ���Ϊԭ�ӡ�
��1�������������ɵ����ӻ������ˮ��Ӧ����һ�ֿ�ȼ�����壬��Ӧ�Ļ�ѧ����ʽ��______________________________��
��2�����ڸ���ʱ��һ�ֻ�ԭ�������û�ѧ����ʽ��ʾ���ڹ�ҵ�ϵ�һ����Ҫ��;��
________________________________________��
��3����һ�������£�����O2��Ӧ�Ļ�ѧ����ʽ��______________________________��
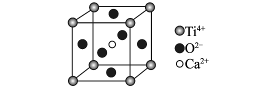
��4������Ԫ�ط�����__________������ԭ�ӽṹʾ��ͼΪ__________��
��5������������ľ���ṹ��__________�ľ���ṹ���ơ�
��1�������������ɵ����ӻ������ˮ��Ӧ����һ�ֿ�ȼ�����壬��Ӧ�Ļ�ѧ����ʽ��______________________________��
��2�����ڸ���ʱ��һ�ֻ�ԭ�������û�ѧ����ʽ��ʾ���ڹ�ҵ�ϵ�һ����Ҫ��;��
________________________________________��
��3����һ�������£�����O2��Ӧ�Ļ�ѧ����ʽ��______________________________��
��4������Ԫ�ط�����__________������ԭ�ӽṹʾ��ͼΪ__________��
��5������������ľ���ṹ��__________�ľ���ṹ���ơ�
��1��CaC2+2H2O====C2H2��+Ca(OH)2
��2�����ԡ���ֻҪ�����Ĵ𰸷�������Ҫ���ɸ��֣�
��3��N2+O2 2NO
2NO
��4��Si��
��5�����ʯ
��2�����ԡ���ֻҪ�����Ĵ𰸷�������Ҫ���ɸ��֣�
��3��N2+O2
 2NO
2NO��4��Si��

��5�����ʯ
��������ڣ�1�������е���Ϣ��֪CaC2��H2O��Ӧ��ȷ����ΪC2-2����˿���ȷ���ס��ҡ���������Ϊ14�������ӣ���Ϊԭ�ӣ���ȷ��ΪSi ,���ڸ�����Ϊ��ҵ��һ����Ҫ�Ļ�ԭ������ȷ��ΪCO����ΪN2��SiO2Ϊԭ�Ӿ��壬����ʯ�ľ���ṹ���ơ�

��ϰ��ϵ�д�
�����Ŀ