��Ŀ����
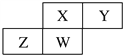
����Ŀ��������Ԫ��X��Y��Z��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������Wԭ�ӵ�����������������������������
��ش��������⣺
��1��Zλ�����ڱ��е�λ��______________��
��2��X��YԪ�ص��⻯���У����ȶ��Ե���________���ѧʽ����
��3��X����̬�⻯����������������Ӧ��ˮ���ﷴӦ���ɵĻ������д��ڵĻ�ѧ������Ϊ_________��
��4����д��X������������Ӧˮ�����ϡ��Һ���������۷�Ӧ�����ӷ�Ӧ����ʽ��______________��
���𰸡� �������ڵڢ�A�� H2O ���Ӽ������ۼ� Fe+4H++NO3-��Fe3++NO��+2H2O
��������������������Ԫ��X��Y��Z��W��Ԫ�����ڱ��е����λ����ͼ��ʾ������Wԭ�ӵ�����������������������������Wλ�ڵ������ڣ���W��P������X��N��Y��O��Z��Si�����Ԫ�������ɽ����
������������Ϸ�����֪X��N��Y��O��Z��Si��W��P����
��1��Z��Si��λ�����ڱ��е�λ��Ϊ�������ڵڢ�A�塣
��2���ǽ�����Խǿ���⻯��Խ�ȶ����ǽ�������O��N����X��YԪ�ص��⻯���У����ȶ��Ե���H2O��
��3��X����̬�⻯�ﰱ����������������Ӧ��ˮ�������ᷴӦ���ɵĻ�����������泥����д��ڵĻ�ѧ������Ϊ���Ӽ������ۼ���
��4��X������������Ӧˮ�����ϡ��Һ��ϡ���ᣬ���������۷�Ӧ�����ӷ�Ӧ����ʽΪFe+4H++NO3-��Fe3++NO��+2H2O��


