��Ŀ����
ij��Һ���ܺ���Cl-��SO42-��CO32-��NH4+��Fe3+��Fe2+��Al3+��Na+��ijͬѧΪ��ȷ����ɷ֣�ȡ������Һ����Ʋ����������ʵ�飺
�ɴ˿�֪ԭ��Һ�У�������

�ɴ˿�֪ԭ��Һ�У�������
| A��ԭ��Һ��c��Fe3+��=0.2mol?L-1 |
| B����Һ��������4�����Ӵ��ڣ�����Cl-һ�����ڣ���c��Cl-����0.2mol?L-1 |
| C��SO42-��NH4+��Na+һ�����ڣ�CO32-��Al3+һ�������� |
| D��Ҫȷ��ԭ��Һ���Ƿ���Fe2+�������Ϊ��ȡ����ԭ��Һ���Թ��У�����������ˮ���������ټ�KSCN��Һ����Һ��Ѫ��ɫ������Fe2+ |
���㣺���ʷ��롢�ᴿ��ʵ�鷽�����,���ʵķ��롢�ᴿ�Ļ�������ѡ����Ӧ��
ר�⣺ʵ�������
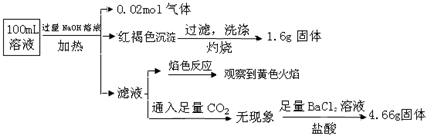
�������������NaOH��Һ�����ȣ��õ�0.02mol���壬������Ϊ������ԭ��Һ��һ������0.02molNH4+��
�����ĺ��ɫ����Ϊ����������1.6g����Ϊ�����������������������ʵ���Ϊ0.01mol����ԭ��Һ�к���0.02mol��Ԫ�أ�����ΪFe3+��Fe2+��ԭ��Һ��һ��û��CO32-��
��Һͨ�������̼��������ԭ��Һ��һ��������Al3+��
4.66g����������Ĺ���Ϊ���ᱵ�����ᱵ�����ʵ���Ϊ0.02mol��ԭ��Һ�к���0.02mol��������ӣ�
��ɫ��ӦΪ��ɫ������Һ�д��������ӣ����ڼ���������������Һ�����ж�ԭ��Һ���Ƿ��������ӣ�
������Һ�������ж��Ƿ���������ӣ�
�����ĺ��ɫ����Ϊ����������1.6g����Ϊ�����������������������ʵ���Ϊ0.01mol����ԭ��Һ�к���0.02mol��Ԫ�أ�����ΪFe3+��Fe2+��ԭ��Һ��һ��û��CO32-��
��Һͨ�������̼��������ԭ��Һ��һ��������Al3+��
4.66g����������Ĺ���Ϊ���ᱵ�����ᱵ�����ʵ���Ϊ0.02mol��ԭ��Һ�к���0.02mol��������ӣ�
��ɫ��ӦΪ��ɫ������Һ�д��������ӣ����ڼ���������������Һ�����ж�ԭ��Һ���Ƿ��������ӣ�
������Һ�������ж��Ƿ���������ӣ�
���
�⣺�������NaOH��Һ�����ȣ��õ�0.02mol���壬������Ϊ������ԭ��Һ��һ������0.02molNH4+��
�����ĺ��ɫ����Ϊ����������1.6g����Ϊ�����������������������ʵ���Ϊ0.01mol����ԭ��Һ�к���0.02mol��Ԫ�أ�����ΪFe3+��Fe2+��ԭ��Һ��һ��û��CO32-��
��Һͨ�������̼��������ԭ��Һ��һ��������Al3+��
��ɫ��ӦΪ��ɫ������Һ��һ�����������ӣ����ڼ�����NaOH�����ж�ԭ��Һ���Ƿ���Na+��
4.66g����������Ĺ���Ϊ���ᱵ�����ᱵ�����ʵ���Ϊ0.02mol����ԭ��Һ�к���0.02mol��������ӣ�
�ݵ���غ㣬ԭ��Һ��һ����Cl-�����ʵ�������Ϊ 0.02mol��2+0.02mol-0.02mol��2=0.02mol��
A���������Ϸ�����֪��ԭ��Һ�к���0.02mol��Ԫ�أ����жϴ��ڵ��������ӻ����������ӣ���A����
B���������Ϸ�����ԭ��Һ��һ������0.02molNH4+��0.02molSO42-��0.02molFe3+��Fe2+�е�һ�֣�����Ԫ��ȫ��Ϊ��������ʱ��������������ɵ����ʵ�����С��������������ʵ�������Ϊ��0.02mol��2+0.02mol=0.06mol��������ɵ����ʵ���Ϊ��0.02mol��2=0.04mol��������Һ�����Կ�֪��ԭ��Һ��һ������Cl-����c��Cl-����
=0.2 mol?L-1����B��ȷ��
C���������Ϸ�����֪��ԭ��Һ��һ������SO42-��NH4+��Cl-��ֻ�Ǵ���Fe3+��Fe2+�е�һ�֣������ӵ����ʵ���Ϊ0.02mol��һ��������CO32-��Al3+�����ڵ�һ���м���������������Һ�������������ӣ���ȷ��ԭ��Һ���Ƿ��������ӣ���C����
D��������������ʱ��ȡ����ԭ��Һ���Թ��У���KSCN��Һ����ʱ��Һ�����������ɫ��Ȼ���ټ���������ˮ����Һ��Ѫ��ɫ������Fe2+����������������軯����Һ����ʾ��ɫ������֤��ԭ��Һ���Ƿ����������ӣ���D����
��ѡB��
�����ĺ��ɫ����Ϊ����������1.6g����Ϊ�����������������������ʵ���Ϊ0.01mol����ԭ��Һ�к���0.02mol��Ԫ�أ�����ΪFe3+��Fe2+��ԭ��Һ��һ��û��CO32-��
��Һͨ�������̼��������ԭ��Һ��һ��������Al3+��
��ɫ��ӦΪ��ɫ������Һ��һ�����������ӣ����ڼ�����NaOH�����ж�ԭ��Һ���Ƿ���Na+��
4.66g����������Ĺ���Ϊ���ᱵ�����ᱵ�����ʵ���Ϊ0.02mol����ԭ��Һ�к���0.02mol��������ӣ�
�ݵ���غ㣬ԭ��Һ��һ����Cl-�����ʵ�������Ϊ 0.02mol��2+0.02mol-0.02mol��2=0.02mol��
A���������Ϸ�����֪��ԭ��Һ�к���0.02mol��Ԫ�أ����жϴ��ڵ��������ӻ����������ӣ���A����
B���������Ϸ�����ԭ��Һ��һ������0.02molNH4+��0.02molSO42-��0.02molFe3+��Fe2+�е�һ�֣�����Ԫ��ȫ��Ϊ��������ʱ��������������ɵ����ʵ�����С��������������ʵ�������Ϊ��0.02mol��2+0.02mol=0.06mol��������ɵ����ʵ���Ϊ��0.02mol��2=0.04mol��������Һ�����Կ�֪��ԭ��Һ��һ������Cl-����c��Cl-����
| 0.06mol-0.04mol |
| 0.1L |
C���������Ϸ�����֪��ԭ��Һ��һ������SO42-��NH4+��Cl-��ֻ�Ǵ���Fe3+��Fe2+�е�һ�֣������ӵ����ʵ���Ϊ0.02mol��һ��������CO32-��Al3+�����ڵ�һ���м���������������Һ�������������ӣ���ȷ��ԭ��Һ���Ƿ��������ӣ���C����
D��������������ʱ��ȡ����ԭ��Һ���Թ��У���KSCN��Һ����ʱ��Һ�����������ɫ��Ȼ���ټ���������ˮ����Һ��Ѫ��ɫ������Fe2+����������������軯����Һ����ʾ��ɫ������֤��ԭ��Һ���Ƿ����������ӣ���D����
��ѡB��
���������⿼�����ʷ��롢�ᴿʵ�鷽������ƣ�Ϊ��Ƶ���㣬����ʵ�������е��Լ��������ķ�Ӧ�����뷽��Ϊ���Ĺؼ������ط���������ʵ���������ۺϿ��飬��Ŀ�ѶȲ���ע�������Һ�������ж������Ӵ��ڵķ�����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
����˵������ȷ���ǣ�������
| A����ϩ�������屽�����е�����ԭ�Ӿ���ƽ�� |
| B��ˮ������1000�����ϲŷֽ�����Ϊˮ���Ӽ��������� |
| C��������Ũ�����Ũ������ʹ�������ۻ������Կ��������۹�����Ũ���ᡢŨ���� |
| D���ܷ���������Ӧ���л���ṹ��һ������-CHO |
�����һ�������������˹��ϳɵ��л����ǣ�������
| A���Ҵ� | B��ʳ�� | C������ | D������ |
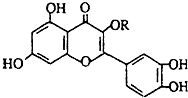 ��Ȼά����P���ṹ��ͼ�������ڻ��������У�����һ��Ӫ��������������ά����P������������ǣ�������
��Ȼά����P���ṹ��ͼ�������ڻ��������У�����һ��Ӫ��������������ά����P������������ǣ�������| A��1molά����P��һ�������������Ժ�7mol H2��Ӧ |
| B����������������� |
| C��ά����P����FeCl3��Һ������ɫ��Ӧ |
| D��1molά����P���Ժ�4mol NaOH��Ӧ |
���й��ڼ۲���ӶԻ���ģ�ͣ�VSEPRģ�ͣ��������в���ȷ���ǣ�������
| A��VSEPRģ�Ϳ�����Ԥ����ӵ�����ṹ |
| B�������м۵��Ӷ���ų�����˷��ӵĿռ�ṹ |
| C�������м���Խ�۵��Ӷ���ų���ԽС������Խ�ȶ� |
| D������ԭ���ϵŵ��ӶԲ����뻥���ų� |
A��B��ͬһ�����ڵ����ֽ���Ԫ�أ���aAm+���Ӱ뾶����bBn+���Ӱ뾶�������бȽ���ȷ���ǣ�������
| A�������ԣ�A��B |
| B�������ԣ�Am+��Bn+ |
| C��m��n |
| D��a��b |
��NAΪ����٤��������ֵ������������ȷ���ǣ�������
| A��22.4L�����к���C-H������ĿΪ4NA |
| B��46g NO2��N2O4�Ļ�������к���ԭ������Ϊ3NA |
| C��25�棬pH=13��NaOH��Һ�к���OH-����ĿΪ0.1NA |
| D��Na2O2������H2O��Ӧ��������0.1mol O2��ת�Ƶ��ӵ���ĿΪ0.4NA |
�����л�ʵ�������������ȷ���ǣ�������
| A������ϩ����Ȳ����ֱ�ͨ��������Ȼ�̼��Һ��ǰ����ɫ�����߲���ɫ |
| B�������������Ȳ�ɽ����߷ֱ�ͨ�����Ը��������Һ�� |
| C������ʹ��ˮ��ɫ����Ϊ���߷�Ӧ�������屽 |
| D����ҵ���Ʊ�����ϩ������Ȳ��һ�����������Ȼ��ⷢ��ȡ����Ӧ |