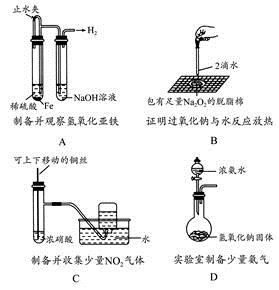
��Ŀ����
B�� [ʵ�黯ѧ]
���������Ƽ�ȩ(NaHSO2��HCHO��2H2O)��ӡȾ��ҽҩ�Լ�ԭ���ܹ�ҵ��Ӧ�ù㷺����Na2SO3��SO2��HCHO ��п��Ϊԭ���Ʊ����������Ƽ�ȩ��ʵ�鲽�����£�
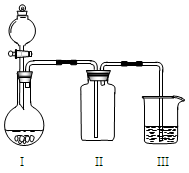
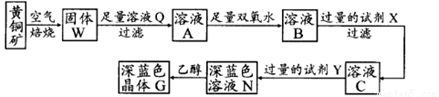
����1������ƿ��(װ����ͼ ��ʾ) ����һ����Na2SO3��ˮ�������ܽ⣬����ͨ��SO2������ҺpH ԼΪ4���Ƶ�NaHSO3��Һ������2����װ��A �е����ܻ�����Ƥ��������ƿ�м����Թ�����п�ۺ�һ������ȩ��Һ����80 ~ 90���£���ӦԼ3h����ȴ�����£����ˡ�����3������Һ�������Ũ������ȴ�ᾧ��

(1)װ��B ���ձ���Ӧ�������Һ�� ��
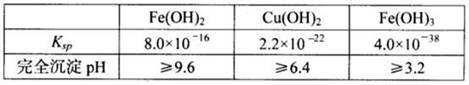
(2)�ٲ���2 �У���Ӧ���ɵ�Zn(OH)2�Ḳ����п�۱�����ֹ��Ӧ���У���ֹ���������Ĵ�ʩ��
�����������л�������Ҫ���ʳ�H2O ��� (�ѧʽ)��
(3)�ٳ���װ������������������ѹϵͳ��� �� (����������)������������Ҫ�ɷ��� �� (�ѧʽ)��
(4)���������Ƽ�ȩ����ǿ��ԭ�ԣ�����120�����Ϸ����ֽ⡣����3 �в��ڳ�������������Ũ����ԭ���� ��
���������Ƽ�ȩ(NaHSO2��HCHO��2H2O)��ӡȾ��ҽҩ�Լ�ԭ���ܹ�ҵ��Ӧ�ù㷺����Na2SO3��SO2��HCHO ��п��Ϊԭ���Ʊ����������Ƽ�ȩ��ʵ�鲽�����£�
����1������ƿ��(װ����ͼ ��ʾ) ����һ����Na2SO3��ˮ�������ܽ⣬����ͨ��SO2������ҺpH ԼΪ4���Ƶ�NaHSO3��Һ������2����װ��A �е����ܻ�����Ƥ��������ƿ�м����Թ�����п�ۺ�һ������ȩ��Һ����80 ~ 90���£���ӦԼ3h����ȴ�����£����ˡ�����3������Һ�������Ũ������ȴ�ᾧ��

(1)װ��B ���ձ���Ӧ�������Һ�� ��
(2)�ٲ���2 �У���Ӧ���ɵ�Zn(OH)2�Ḳ����п�۱�����ֹ��Ӧ���У���ֹ���������Ĵ�ʩ��
�����������л�������Ҫ���ʳ�H2O ��� (�ѧʽ)��
(3)�ٳ���װ������������������ѹϵͳ��� �� (����������)������������Ҫ�ɷ��� �� (�ѧʽ)��
(4)���������Ƽ�ȩ����ǿ��ԭ�ԣ�����120�����Ϸ����ֽ⡣����3 �в��ڳ�������������Ũ����ԭ���� ��

��1��װ��B��������ʣ���SO2β����Ӧ����NaOH��Һ���ա���2���ٷ�ֹZn(OH)2������п�۵ı�����ÿ��ٽ���ķ������ڷ�Ӧ���еļ�ȩ�е�ϵͣ����лӷ��ԣ���������������ȴ����3������װ�û���������©��������ƿ�������к������ɵ�Zn(OH)2�Լ�����δ��Ӧ��п����4��������֪�����������Ƽ�ȩ����ǿ��ԭ�ԣ����������Ŀ���Ƿ�ֹ�䱻�����е�����������
�����㶨λ����ѧʵ��ģ��
�����㶨λ����ѧʵ��ģ��

��ϰ��ϵ�д�
�����Ŀ

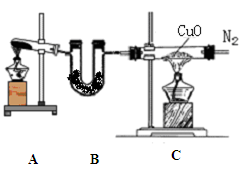
 ������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00ml����ش��������⣺
������ƿ�У���0.05mol/L������Һ���еζ������ı���Һ20.00ml����ش��������⣺
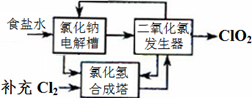
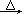 2ClO2��+2Na2SO4��H2O
2ClO2��+2Na2SO4��H2O H++OH-�� ________________�������ӷ���ʽ��ʾ��.
H++OH-�� ________________�������ӷ���ʽ��ʾ��.