��Ŀ����
A��B�����л�������л��ϳɵ��м��壬����A�ķ���ʽΪC4H7O2Br��B�����к�2����ԭ�ӣ���ȼ�ղ���n��CO2����n��H2O��=2��1������ͼ����B����Է�������Ϊ188��A��B��������ת����ϵ��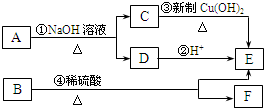
��֪����һ��̼ԭ�������������ǻ�ʱ����������ת����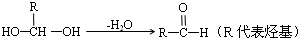
��ͬһ��̼ԭ������������˫���Ľṹ���ȶ���
��ش�
��1��C�����Ƶ�������ͭ��Ӧ�Ļ�ѧ����ʽ�� ��
��2��A�Ľṹ��ʽ�� ��
��3��B�ķ���ʽ�� ��
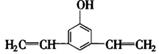
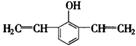
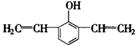
��4��F���������ص㣺�پ��������ԣ��ں˴Ź�����������ʾ�������շ壻�۱����ϵ�һ�ȴ���ֻ�����֣��ܳ������⣬������������״�ṹ��д���������������Ҿ����ȶ��ṹ����������ͬ���칹��Ľṹ��ʽ�� �� ��
��1��CH3CHO+2Cu(OH)2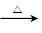 CH3COOH+Cu2O��+2H2O��2�֣�
CH3COOH+Cu2O��+2H2O��2�֣�
��2��CH3COOCHBrCH3��2�֣�����3��C12H12O2��1�֣�
��4��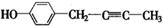 ��
��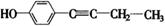 ��
�� ��
�� ����д������ÿ��2�֣�
����д������ÿ��2�֣�
�������������C��D����ת����E��˵��C��D���е�̼ԭ������ȣ�������2��̼ԭ�ӣ�C�������Ʊ�������ͭ��Ӧ��CΪCH3CHO��EΪCH3COOH����DΪCH3COONa�����A�ķ���ʽ�Լ������Ϣ��֪AΪCH3COOCHBrCH3��B�����к�2����ԭ�ӣ���ȼ�ղ���n��CO2����n��H2O��=2��1��˵��������C��Hԭ������ȣ�����ͼ����B����Է�������Ϊ188�������ʽΪCnHnO2����13n+32=188��n=12�������ʽΪC12H12O2����F�ķ���ʽΪC10H10O����=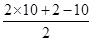 =6��
=6��
��1��CΪCH3CHO����������ͭ��Ӧ�ķ���ʽΪCH3CHO+2Cu(OH)2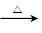 CH3COOH+Cu2O��+2H2O��
CH3COOH+Cu2O��+2H2O��
��2�������Ϸ�����֪AΪCH3COOCHBrCH3��
��3��B�ķ���ʽ��C12H12O2����
��4��F�ķ���ʽΪC10H10O����=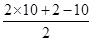 =6���پ��������ԣ�Ӧ���з��ǻ���
=6���پ��������ԣ�Ӧ���з��ǻ���
�ں˴Ź�����������ʾ�������շ壻�۱����ϵ�һ�ȴ���ֻ�����֣�ӦΪ�Գƽṹ���ܳ������⣬������������״�ṹ��������Ӧ����1��C��C��2��C=C������ܵĽṹΪ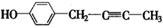 ��
�� ��
�� ��
��
���㣺���鿼���л�����ƶϡ�

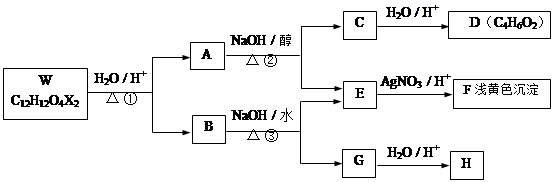

 ����Ӧ�ܵĻ�ѧ����ʽ�� ������ ��
����Ӧ�ܵĻ�ѧ����ʽ�� ������ ��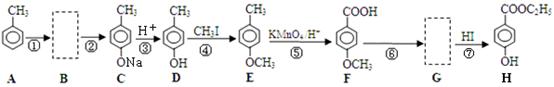
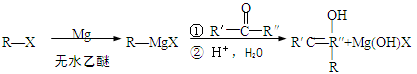

 ���ۺ϶��ɣ���һ�ֶ�����ϣ��㷺Ӧ����ʳƷ��װ�������豸�������ճ����������С�д���� D�ͱ�Ϊ��Ҫԭ���Ʊ�����ϩ��
���ۺ϶��ɣ���һ�ֶ�����ϣ��㷺Ӧ����ʳƷ��װ�������豸�������ճ����������С�д���� D�ͱ�Ϊ��Ҫԭ���Ʊ�����ϩ�� B ����
B ����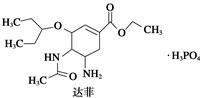


 )�������ҹ�ʢ���İ˽������С�����ç�������ʵ�����������˵����ȷ���ǣ� ��
)�������ҹ�ʢ���İ˽������С�����ç�������ʵ�����������˵����ȷ���ǣ� ��


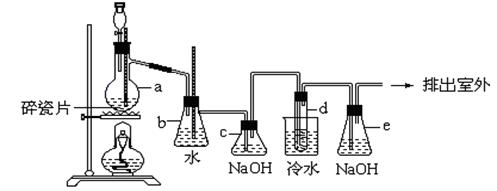
 �ṹ�����ʣ� ��
�ṹ�����ʣ� ��
