��Ŀ����
����Ŀ�����ɰ(SiC)���� SiO2 ��̼��һ�������·�Ӧ�Ƶã��ڷ�Ӧ����ʽΪ SiO2+3C![]() SiC+2CO�����������й�������ɰ��˵������ȷ����
SiC+2CO�����������й�������ɰ��˵������ȷ����
A. �÷�Ӧ�е��������� SiO2����ԭ��Ϊ C
B. �÷�Ӧ˵����C �Ļ�ԭ��С��SiC
C. �÷�Ӧ��ת�Ƶĵ�����Ϊ 12e-
D. �÷�Ӧ�еĻ�ԭ������ SiC������������ CO�������ʵ���֮��Ϊ 1:2
���𰸡�D
��������
�ڷ�Ӧ����ʽΪSiO2+3C![]() SiC+2CO���У�3mol̼����2mol̼�Ļ��ϼ����ߣ�������������ԭ����1mol̼�Ļ��ϼ۽��ͣ�SiC��̼Ϊ��4�ۣ�������ԭ��������������A����ԭ���Ļ�ԭ�Դ��ڻ�ԭ����ɷ���ʽ��֪��C�Ļ�ԭ�Դ���SiC��B�����ڸ÷�Ӧ��ת�Ƶĵ�����Ϊ4e-��C���÷�Ӧ�еĻ�ԭ������SiC������������CO�������ʵ���֮��Ϊ1:2��D��ȷ����ѡD��
SiC+2CO���У�3mol̼����2mol̼�Ļ��ϼ����ߣ�������������ԭ����1mol̼�Ļ��ϼ۽��ͣ�SiC��̼Ϊ��4�ۣ�������ԭ��������������A����ԭ���Ļ�ԭ�Դ��ڻ�ԭ����ɷ���ʽ��֪��C�Ļ�ԭ�Դ���SiC��B�����ڸ÷�Ӧ��ת�Ƶĵ�����Ϊ4e-��C���÷�Ӧ�еĻ�ԭ������SiC������������CO�������ʵ���֮��Ϊ1:2��D��ȷ����ѡD��

����Ŀ��ʵ�������̷�(FeSO4��7H2O)�Ʊ���Ѫ���ʰ�������[(NH2CH2COO)2Fe] �й������������£�
�ʰ���(NH2CH2COOH) | ������ | �ʰ������� |
������ˮ�������Ҵ������Ի����� | ������ˮ���Ҵ�����ǿ���Ժͻ�ԭ�� | ������ˮ���������Ҵ� |
ʵ����̣�
��.���ƺ�0.10mol FeSO4���̷���Һ��
��.�Ʊ�FeCO3�������ƺõ��̷���Һ�У���������200mL 1.1mol��L-1NH4HCO3��Һ���ӱ߽��裬��Ӧ��������˲�ϴ�ӳ�����
��.�Ʊ�(NH2CH2COO)2Fe��ʵ��װ������ͼ���гֺͼ���������ʡ�ԣ�����ʵ���õ��ij����ͺ�0.20 mol�ʰ����ˮ��Һ��Ϻ����C�У�Ȼ������A�еķ�Ӧ��C�п����ž������ŵ�����������Һ�����ȡ���Ӧ��������ˣ���Һ�������ᾧ�����ˡ�ϴ�ӡ�����õ���Ʒ��
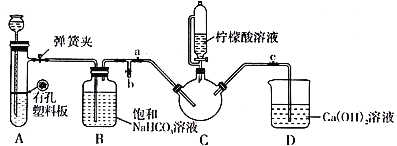
�ش��������⣺
��1��ʵ��I�У�ʵ���������̷���Һʱ��Ϊ��ֹFeSO4���������ʣ�Ӧ������Լ�Ϊ_______��д��ѧʽ�����ٵμ�����ϡ�����������__________________________��
��2��ʵ��II�У����ɳ��������ӷ���ʽΪ__________________________��
��3��ʵ����У���C��ʢ��������Һ����������Ϊ___________________��
��װ��A����ʢ�ŵ�ҩƷ��___________________��������ţ�
A.Na2CO3��ϡH2SO4 B.CaCO3��ϡH2SO4 C.CaCO3��ϡ����
��ȷ��C�п����ž���ʵ��������____________________��
�ܼ�����������Һһ����ɵ�����Һ��pH�ٽ�FeCO3�ܽ⣬��һ��������________________��
��ϴ��ʵ����еõ��ij�������ѡ�õ����ϴ���Լ���___________________��������ţ�
A.��ˮ B.�Ҵ���Һ C.��������Һ
������Ʒ������Ϊ17.34g,�����Ϊ________%��