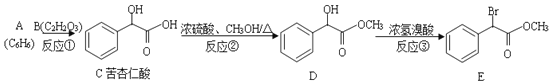
��Ŀ����
����Ŀ��ij��ȤС�����SO2ʵ�鷽���������»�ѧʵ�顣
��.ʵ�鷽��һ
(1)��SO2ͨ��ˮ���γɡ�SO2������H2SO3��Һ����ϵ������ϵ�д��ڶ������Ԫ�ص�ƽ�⣬д������1��ƽ�ⷽ��ʽ��________________________________________��
(2)��֪�����Ѿ���������SO2��������������[�ҹ����ұ�(GB2760��2014)�涨���Ѿ���SO2�IJ�������0.25 g��L��1]��
����SO2��Ư���Լ��ɰ����Ѿ�(Һ��Ϊ��ɫ)�е�SO2��H2SO3�������ͼ1��ʵ�飺
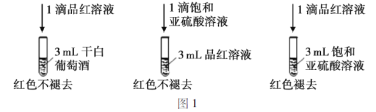
ʵ����ۣ��ɰ����ѾƲ���ʹƷ����Һ��ɫ��ԭ��Ϊ��_____________��
��.ʵ�鷽����
��ͼ2��ʵ���ҽ��ж��������Ʊ�������ʵ������װ�ã����̶ֹ�װ��δ������
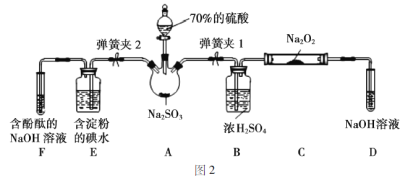
(1)�رյ��ɼ�2�����ɼ�1��ע����������û������ƿ�й��壬����SO2��Na2O2��Ӧ�Ƿ����������ɵķ�����_________________________��
(2)װ��D��ʢ��NaOH��Һ��������______________________��
(3)�رյ��ɼ�1���ɼ�2�������������E��F�У���˵��I����ԭ������SO2������Ϊ_____________��������Ӧ�����ӷ���ʽ��______________��
��.ʵ�鷽����
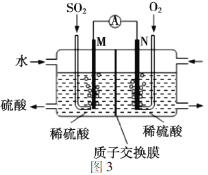
�õ绯ѧ��ģ�ҵ����SO2�������Ṥҵβ���е�SO2ͨ��ͼ3װ��(�缫��Ϊ���Բ���)����ʵ�飬�������Ʊ����ᣬͬʱ��õ��ܡ�M�������ĵ缫��ӦʽΪ_______��
���𰸡�SO2(g)![]() SO2(aq)��SO2+H2O
SO2(aq)��SO2+H2O![]() H2SO3��H2SO3
H2SO3��H2SO3![]() H++HSO3-��HSO3-
H++HSO3-��HSO3-![]() H++SO32- �ɰ��ж�������������Ậ��̫�� �������ǵ�ľ������D�Թܿڴ�����ľ���Ƿ�ȼ ����δ��Ӧ��SO2����ֹ��Ⱦ���� E����Һ��ɫ��ɫ SO2+I2+2H2O=2I-+SO42-+4H+ SO2+ 2H2O-2e-=SO42-+4H+
H++SO32- �ɰ��ж�������������Ậ��̫�� �������ǵ�ľ������D�Թܿڴ�����ľ���Ƿ�ȼ ����δ��Ӧ��SO2����ֹ��Ⱦ���� E����Һ��ɫ��ɫ SO2+I2+2H2O=2I-+SO42-+4H+ SO2+ 2H2O-2e-=SO42-+4H+
��������
ʵ���ҽ��ж��������Ʊ�������ʵ�飬Ũ������������Ʒ�Ӧ��H2SO4+Na2SO3=Na2SO4+ H2O + SO2���������۵ĵ�Һ���ڼ������������ⵥ�ʵ�������ԭ��Ӧ������̪���������Ƽ��������������ԣ�ϴ��ƿ��Ũ�������ڸ������壬����Ķ���������������Ʒ�Ӧ�����β���������������ա�
��.(1)��SO2ͨ��ˮ���γɡ�SO2������H2SO3��Һ����ϵ�����ڶ�����������ˮ��ƽ�⣬��������ˮ�еĶ�������ƽ�⣬��ƽ���ϵ����SO2(g)![]() SO2(aq)��SO2+H2O
SO2(aq)��SO2+H2O![]() H2SO3��H2SO3
H2SO3��H2SO3![]() H++HSO3-��HSO3-
H++HSO3-��HSO3-![]() H++SO32-��
H++SO32-��
(2)��������ʵ��Աȣ�������������Һ�������ʱ��Ʒ����ɫ������ͬ����ĸɰ���Ȼ����ɫ����˵���ɰ����ѾƲ���ʹƷ����Һ��ɫ��ԭ��Ϊ�ɰ��ж�������������Ậ��̫�٣�
��.(1)��������ȼ�����壬�����Ƿ����������ɵķ����ǽ������ǵ�ľ������D�Թܿڴ�����ľ���Ƿ�ȼ��
(2)װ��D��ʵ��ĩβ������β���ռ���ʢ��NaOH��Һ������������δ��Ӧ��SO2����ֹ��Ⱦ������
(3)����������ԭ��Ӧԭ������ԭ����ԭ�Դ��ڻ�ԭ�������������ⵥ�ʷ�Ӧ���������ӣ���Һ�еⵥ�ʼ��٣���ɫ����ȥ��������Ӧ�����ӷ���ʽSO2+I2+2H2O=2I-+SO42-+4H+��
��.������������������ԭ��ط�Ӧ������������������������������M������������Ӧ��ʧȥ���ӣ��缫��ӦʽΪSO2+ 2H2O-2e-=SO42-+4H+��

����Ŀ�������ᣨ![]() ������Ҫ�Ļ���ԭ�ϣ���Ӧ��������������Ⱦ�����塢���ܼ������ϼ�ʳƷ��������������Ҳ�����ڸ����豸�ķ������ij��ѧʵ��С����ʵ�������Ա���ȩΪԭ����ȡ���������Ʒ���״���
������Ҫ�Ļ���ԭ�ϣ���Ӧ��������������Ⱦ�����塢���ܼ������ϼ�ʳƷ��������������Ҳ�����ڸ����豸�ķ������ij��ѧʵ��С����ʵ�������Ա���ȩΪԭ����ȡ���������Ʒ���״���![]() ����ʵ������:
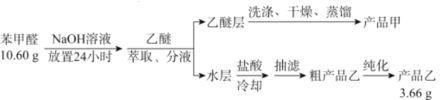
����ʵ������:
��֪���� ��
��![]() ��
�� ��R��R1��ʾ��������ԭ�ӣ�
��R��R1��ʾ��������ԭ�ӣ�
��������ʵIJ����������ʼ�����
���� | ����ܶ� | �۵�/�� | �е�/�� | �ܽ�� | |
ˮ | ���� | ||||
����ȩ | 1.04 | ��26 | 179.6 | �� | ���� |
������ | 1.27 | 122.1 | 249 | 25���ܣ�95������ | ���� |
���״� | 1.04 | ��15.3 | 205.7 | �� | ���� |
���� | 0.71 | ��116.3 | 34.6 | ���� | �� |
��ش���������:
��1��������ȡ����Һ����ʱ���ò�������������Ϊ___________����Һʱ�����Ѳ�Ӧ��_______(�����¿ڷų��������Ͽڵ�����)��
��2��ϴ�����Ѳ�ʱ��Ҫ������NaHSO3��Һ��10%Na2CO3��Һ������ˮ����ϴ�ӡ����м���NaHSO3��Һϴ�ӵ���ҪĿ����________________����Ӧ�Ļ�ѧ����ʽΪ___________________________��
��3�������ò�Ʒ��ʱ�������Ƭ��Ŀ��Ϊ_____________������ʱӦ�����¶���____�����ҡ�
A.34.6 B.179.6 C.205.7 D.249
��4���ᴿ�ֲ�Ʒ�һ�ò�Ʒ�ҵĴ�����������Ϊ________________��
��5����ȡ10.60g�ı���ȩ����ʵ�飬������ȡ��Ʒ�ҵ�����Ϊ3.66g�����Ʒ�ҵIJ���Ϊ____________��