��Ŀ����
����Ŀ������ȷ��ʾ���з�Ӧ�����ӷ���ʽ��
A. �ù���ʯ�������չ�ҵβ���е�SO2: Ca2++2OH-+SO2��CaSO3 ��+ H2O
B. ������KMnO4��Һ��H2O2��Ӧ��֤��H2O2���л�ԭ�ԣ�2MnO4��+6H++5H2O2��2Mn2++5O2 ��+8H2O
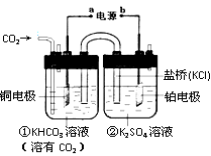
C. ��ͭ���缫���NaCl��Һ:2C1��+2H2O![]() H2��+Cl2��+2OH-
H2��+Cl2��+2OH-
D. �� Fe2O3���뵽 HI ��Һ�У�Fe2O3+ 6H+��2Fe3+ +3H2O
���𰸡�B
�����������������A��ʯ�����е���������ֻ�������ܽ⣬�û�ѧʽ��ʾ����A����B������KMnO4���������ԣ���H2O2��Ӧ����������ԭ��Ӧ���ų���������B��ȷ��C��ͭ�ǻ��Ե缫��������ʱ��ͭҪʧȥ���Ӷ��ܽ⣬��C����D�����ɵ�Fe3+���������ԣ��ܹ����������ӣ���D����ѡB��

 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�����Ŀ������ˮ�к�����ᵼ�����ж���ˮ�����ܽ������Ҫ��As( ��)�������κ�As(V)��������ʽ���ڡ�
(1)������Ϊͬһ����Ԫ�أ���ԭ�ӽṹʾ��ͼΪ___________________��
(2)����Ԫ�������ɣ�����˵����ȷ����____________________(����ĸ����)��
a.����������Ӧˮ��������ԣ�S>P>As b. ԭ�Ӱ뾶��S>P>As c.�ǽ����ԣ�S>P>As
(3)���ڵ���ˮ�������Դ�ж��ּ��裬����һ����Ϊ�Ǹ�����Ļ�����(FeS2)������ΪFe(OH)3��ͬʱ����SO42-���������������������ˮ��FeS2��O2���������ӷ���ʽΪ_________________________��
(4)ȥ��ˮ���е��飬���Ƚ�As(�� )ת��ΪAs(V)��ѡ��NaClO��ʵ�ָ�ת����
��֪��Ͷ��ǰˮ��pH=5.81��0.1 mol/L NaClO��ҺpH=10.5����Һ�����������õ������Ǵ����ᡣ
�о�NaClOͶ������As(��)�����ʵ�Ӱ��õ����½����
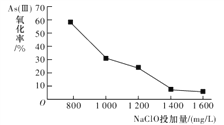
�����˽����ԭ����_________________________��
(5)ǿ�����ӽ�����������������������ʽ���ڵ�As(V)���Ӷ��ﵽȥ��As��Ŀ�ġ�
��֪��һ�������£�As(V)�Ĵ�����ʽ���±���ʾ��
pH | <2 | 2��7 | 7��11 | 11��14 |
������ʽ | H3AsO4 | H2AsO4- | HAsO42- | HAsO42-��AsO43- |
pH=6ʱ��NaClO����������(H3AsO3)�����ӷ���ʽ��_____________________��