��Ŀ����
����Ŀ������Ҫ��ش��������⣺
�� ��Һ�������� �� ��
�� ![]() �۽��ʯ��C60 ��
�۽��ʯ��C60 ��![]() ��
��![]()
�������������У���Ϊͬ���칹�����_____����Ϊͬ�����������_____������ͬ�����ʵ���___________������ţ���
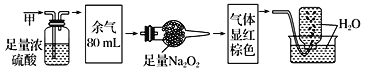
�����������������ʣ�a.NH4Cl b.ˮ�� c.Na2O2 d.�ɱ� e.C60������Ӧ��ĸ���:
��1�����ڷ��Ӿ������____________��
��2�����ڹ��ۻ��������______________��
��3���ۻ�ʱֻ��Ҫ�ƻ����ۼ�����_____________��
��4���Ⱥ������Ӽ��ֺ��й��ۼ�����__________________��
��5������b��e���,Ӳ�Ƚ�С����___________________��
���𰸡��� �� �� de bd b ac e
��������
��Һ������������ͬ�����ʵIJ�ͬ״̬���� ��
�� ![]() ������ʽC6H14����̼����ͬ�����Ի�Ϊͬ���칹�壬�۽��ʯ��C60����ͬ��Ԫ�صIJ�ͬ���ʣ���ͬ�������壬��
������ʽC6H14����̼����ͬ�����Ի�Ϊͬ���칹�壬�۽��ʯ��C60����ͬ��Ԫ�صIJ�ͬ���ʣ���ͬ�������壬��![]() ��
�� ![]() ��������ͬ����������ͬ��������ͬһԪ�صIJ�ͬ���ػ�Ϊͬλ�ء�����������������У���Ϊͬ���칹����Ǣڣ���Ϊͬ����������Ǣۣ�����ͬ�����ʵ��Ǣ١�
��������ͬ����������ͬ��������ͬһԪ�صIJ�ͬ���ػ�Ϊͬλ�ء�����������������У���Ϊͬ���칹����Ǣڣ���Ϊͬ����������Ǣۣ�����ͬ�����ʵ��Ǣ١�
����a.NH4Cl�������Ӿ��壬���й��ۼ������Ӽ����������ӻ�����ۻ�ʱ�ƻ����Ӽ���
b.ˮ������ԭ�Ӿ��壬ֻ���й��ۼ������ڹ��ۻ�����ۻ�ʱ�ƻ����ۼ���
c.Na2O2�������Ӿ��壬�������Ӽ����ۼ����������ӻ�����ۻ�ʱ�ƻ����Ӽ���
d.�����ڷ��Ӿ��壬������ֻ���й��ۼ������ڹ��ۻ�����ۻ�ʱ�ƻ����»����������
e.C60���ڷ��Ӿ��壬������ֻ���й��ۼ������ڵ��ʣ��ۻ�ʱ�ƻ����»�����
ԭ�Ӿ���Ӳ�ȴ������Ӿ��壬���Ӿ�����ڷ��Ӿ��壬ˮ����Ӳ�ȴ���C60��Ӳ�ȡ�
�����1�����ڷ��Ӿ������de��
��2�����ڹ��ۻ��������bd��
��3���ۻ�ʱֻ��Ҫ�ƻ����ۼ�����b��
��4���Ⱥ������Ӽ��ֺ��й��ۼ�����ac��
��5������b��e��ȣ�Ӳ�Ƚ�С����e��

����Ŀ���������״���;����㷺��Խ��Խ�����̼ҵĹ�ע����ҵ�ϼ״��ĺϳ�;�����ֶ���������ʵ������ģ��״��ϳɷ�Ӧ,��2 L�ܱ������ڣ�400 ��ʱ������ӦCO(g)+2H2(g) ![]() CH3OH(g)����ϵ��n(CO)��ʱ��ı仯�����
CH3OH(g)����ϵ��n(CO)��ʱ��ı仯�����
ʱ��(s) | 0 | 1 | 2 | 3 | 5 |
n(CO)(mol) | 0.020 | 0.011 | 0.008 | 0.007 | 0.007 |
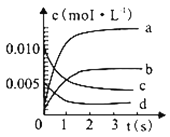
��1��ͼ�б�ʾCH3OH �ı仯��������_______��
��2�����д�ʩ������߷�Ӧ���ʵ���_________(������Ӧ��ĸ���)��
a.�����¶� b.������� c.����ѹǿ d.��ʱ�����CH3OH
��3������������˵����Ӧ�ﵽƽ��״̬����__________(������Ӧ��ĸ���)��
a.CO��H2��Ũ�ȱ��ֲ��� b.v(H2)=2 v(CO)
c.CO�����ʵ����������ֲ��� d.�����������ܶȱ��ֲ���
e.ÿ����1molCH3OH��ͬʱ��2molH-H������
��4��CH3OH��O2�ķ�Ӧ�ɽ���ѧ��ת��Ϊ���ܣ��乤��ԭ����ͼ��ʾ��ͼ��CH3OH��__________����A��B��ͨ�룬b���ĵ缫��Ӧʽ��__________��

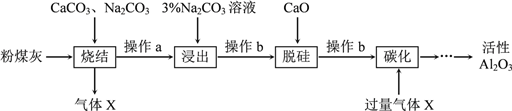
����Ŀ��̼���һ�ֽϳ�ʹ�õĻ��ʣ����ڳ������ֽ⣮ij��ѧ��ȤС���̼淋ijɷִ������ʣ�ʱ��������̽����
������ʵ�飩������Һ�е�����������
ȡ������������Թ��У��������ᣬ�����ɵ�����ͨ�����ʯ��ˮ�У��а�ɫ�������ɣ�����ȡ����̼立����Թ��У�����ŨNaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�������ɵ����壬ʯ����ֽ����ɫ��
��1������ʵ�������Ʋ�̼��������������ӿ�����_________��__________��
��2������ʵ������̼�������NaOH��Һ���ȷ�Ӧ�����ӷ���ʽ������________________��
���������飩�ⶨ̼���CԪ�غ�NԪ�������ȣ�����ȤС��ȷ��ȡag̼泥�����ʹ֮�ֽ⣬���Ѳ���ͨ���ʯ���У���ͼ1��ʾ��
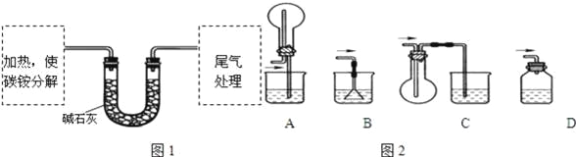
��3��̼粒���Ӧ����________�н��м��ȣ�
A���Թ� | B�������� | C����ƿ | D������ |
��4���Ӱ�ȫ�ĽǶȿ��ǣ�β��������װ�ÿ���ѡ����ͼ2�е�___________��
��5�������պ�û�й�����࣬����U�ι���ʵ��ǰ���������Ϊbg���ɴ˲��NԪ�ص�������_________g��