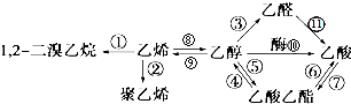
��Ŀ����
10������A��B��C��������A��B�����е�������ԭ�ӱ�C��һ��ȡ��������������ʧȥһ����ԭ�Ӻ�ʣ��IJ��֣�ȡ���IJ����֪����A����ʹ���CCl4��Һ��ɫ����һ�ȴ���ֻ��һ�֣�
��һ������B��ȫȼ�գ�����������ͨ��ʢ��CaCl2�ͼ�ʯ�ҵĸ���ܣ�����������طֱ�Ϊ3.6g��17.6g����26g/mol��M��B����78g/mol��
��CΪ����������ͨ�������Ϊ��̬����ͬ���칹�岻����2�֣����������3�֣�
�ش��������⣺
��1��B�����ʽ��CH��д��B��һ����״ͬ���칹��Ľṹ��ʽCH2=CHC��CH��Ҫ������ԭ����ͬһƽ���ڣ�����̼ԭ�Ӳ�����ͬһֱ���ϣ�
��2��C�ķ���ʽΪCH��CH3��3����һ��������������ƴ���Һ�ͼ��������¿��Է�����Ӧ����Ӧ���л����������Ϊ2-��-1-��ϩ��
��3��B���ӵ�������ԭ�ӱ�����ȡ����IJ���Ϊһ������ըҩ����ըҩ��ը�ֽ�����������ʣ�д���ñ�ը��Ӧ�Ļ�ѧ����ʽC4N4O8=4CO2��+2N2����
���� B��ȫȼ�յIJ�����n��CO2����n��H2O��=$\frac{17.6g}{44g/mol}$��$\frac{3.6g}{18g/mol}$=2��1����B����ɿ��Ա�ʾΪ��CH��n������26��M��A����78����26��13n��78�����2��n��6��B��̼ԭ�Ӹ�������Ϊ3��4��5����������ԭ�Ӹ���Ϊż������B�ķ���ʽֻ��ΪC4H4��
C�DZ���������ͨ������³���̬��̼ԭ����ĿС��5����������⣩����ͬ���칹�壬̼ԭ����Ŀ����3��������������֣���CΪ��CH��CH3��3��C���������Ϊ-CH3����A��������Br2��CCl4��Һ����ɫ��˵�������в��������ͼ�����һ�ȴ���ֻ��һ�֣�˵��������Hԭ��Ϊ��ЧHԭ�ӣ��л���A����Ϊ����B�����е�������ԭ�ӱ���C�������������ȡ�����õ��ģ�B�ķ���ʽΪC4H4����AΪ��C4��CH3��4����A�ķ���ʽΪ��C8H12���ݴ˽��н��
��� �⣺B��ȫȼ�յIJ�����n��CO2����n��H2O��=$\frac{17.6g}{44g/mol}$��$\frac{3.6g}{18g/mol}$=2��1����B����ɿ��Ա�ʾΪ��CH��n������26��M��A����78����26��13n��78�����2��n��6��B��̼ԭ�Ӹ�������Ϊ3��4��5����������ԭ�Ӹ���Ϊż������B�ķ���ʽֻ��ΪC4H4��C�DZ���������ͨ������³���̬��̼ԭ����ĿС��5����������⣩����ͬ���칹�壬̼ԭ����Ŀ����3��������������֣���CΪCH��CH3��3��C���������Ϊ-CH3��A��������Br2��CCl4��Һ����ɫ��˵�������в��������ͼ�����һ�ȴ���ֻ��һ�֣�˵��������Hԭ��Ϊ��ЧHԭ�ӣ��л���A����Ϊ����B�����е�������ԭ�ӱ���C�������������ȡ�����õ��ģ�B�ķ���ʽΪC4H4����AΪ��C4��CH3��4����A�ķ���ʽΪ��C8H12��
��1�����ݷ�����֪��B����ʽΪC4H4�����ʽ��CH��B��һ����״ͬ���칹��Ľṹ��ʽ������ԭ����ͬһƽ���ڣ�����̼ԭ�Ӳ�����ͬһֱ���ϣ���������к���1��̼̼˫����1��̼̼��������̼̼������1��C����ṹ��ʽΪ��CH2=CHC��CH��
�ʴ�Ϊ��CH��CH2=CHC��CH��
��2��C�ķ���ʽΪ��CH��CH3��3����һ��������������ƴ���Һ�ͼ��������¿��Է�����Ӧ����Ӧ����Ϊ��CH3C��CH3��=CH2��������Ϊ��2-��-1-��ϩ��
�ʴ�Ϊ��CH��CH3��3��2-��-1-��ϩ��
��3��B�ķ���ʽΪC4H4��B���ӵ�������ԭ�ӱ�����ȡ����IJ���ķ���ʽΪC4N4O8��C4N4O8��һ������ըҩ����ըҩ��ը�ֽ�����������ʣ����Է�Ӧ����Ϊ���������͵������÷�Ӧ����ʽΪ��C4N4O8=4CO2��+2N2����
�ʴ�Ϊ��C4N4O8=4CO2��+2N2����
���� ���⿼���л��ƶϣ���Ŀ�Ѷ��еȣ�ע�����ճ����л���ṹ�����ʣ��ƶ�B�ķ���ʽΪ�����Ĺؼ�����������������ѧ�����Ӧ����ѧ֪ʶ��������������������

 �ƸԹھ��ο���ϵ�д�
�ƸԹھ��ο���ϵ�д�| A�� | ��� | B�� | ���� | C�� | ʯī�� | D�� | ��ʯ�۳� |
| A�� | �Т٢ڢ�������Ӧ�����ڼӳɷ�Ӧ | B�� | ֻ�Тڷ������ǼӾ۷�Ӧ | ||
| C�� | �ܹ���4����Ӧ��������Ӧ | D�� | ��Ӧ�ܢݢޢ���ȡ����Ӧ |
| A�� | ������Ȼ����ȼ�Ͽ�����Ч�ؼ��١�����ЧӦ����������� | |
| B�� | �����Ҵ��ܹ��ܽ�ܶ��л����������Կ����Ҵ���ȡ��ҩ����Ч�ɷ� | |
| C�� | �����ý��ݹ����������Һ�Ĺ���������ˮ�����������ϩ���ﵽ����Ŀ�� | |
| D�� | ���������Ǽ�ȩ��ˮ��Һ������ɱ�������������������ø������ֱ��������ʳƷ |
| A�� | 5�� | B�� | 6�� | C�� | 7�� | D�� | 8�� |
| A�� | Ԫ��W��X���Ȼ����У���ԭ�Ӿ����������8���ӵ��ȶ��ṹ | |
| B�� | Ԫ��X��W�γɵ�ԭ������Ϊ1��1�Ļ������кܶ��֣��Ҷ����ڷǼ��Լ� | |
| C�� | �ǽ����ԣ�Z��X | |
| D�� | Ԫ��Z����Ԫ��Y�γɻ�����Y2Z3���û�������ˮ�� |
| A�� |  | B�� |  | C�� |  | D�� |  |
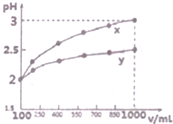 ��1������Ũ��Ϊ0.1mol•-1��������Һ������ڴ�����������Ƣ��Ȼ�泥���������Һ����ˮ�������H+Ũ���ɴ�С��˳���Ǣܢڢۢ٣�����ţ���
��1������Ũ��Ϊ0.1mol•-1��������Һ������ڴ�����������Ƣ��Ȼ�泥���������Һ����ˮ�������H+Ũ���ɴ�С��˳���Ǣܢڢۢ٣�����ţ���