��Ŀ����
19��������һ�ֳ������ᣬ��ṹ��ʽΪ��
��1����������������еĹ����ŵ��������ǻ����Ȼ���
��2��1mol�������������Ʒ�Ӧ���ɵ������ڱ�״���µ����Ϊ22.4L��
��3��д��������Ũ������������Ҵ��ķ�Ӧ��ѧ����ʽ��CH3CH��OH��COOH+C2H5OH$?_{��}^{Ũ����}$CH3CH��OH��COOC2H5+H2O��
��4��д��������������̼���Ʒ�Ӧ�Ļ�ѧ����ʽ��2CH3CH��OH��COOH+Na2CO3��2CH3CH��OH��COONa+H2O+CO2����
���� ��1���÷����к����Ȼ����ǻ���
��2���Ȼ��ʹ��ǻ����ܺ��Ʒ�Ӧ����������
��3��������Ũ����������������Ҵ�����������Ӧ��������������
��4��������ֻ���Ȼ���̼���Ʒ�Ӧ���������ƺͶ�����̼��ˮ��
��� �⣺��1�����ݽṹ��ʽ֪���÷����к����ǻ����Ȼ����ʴ�Ϊ���ǻ����Ȼ���
Ũ����
��2���Ȼ��ʹ��ǻ����ܺ��Ʒ�Ӧ��������������1mol�������ܺ�2molNa��Ӧ����1mol�����������ڱ���µ������22.4L��
�ʴ�Ϊ��22.4L��
��3��������Ũ����������������Ҵ�����������Ӧ����Ӧ����ʽΪCH3CH��OH��COOH+C2H5OH$?_{��}^{Ũ����}$ CH3CH��OH��COOC2H5+H2O��
�ʴ�Ϊ��CH3CH��OH��COOH+C2H5OH$?_{��}^{Ũ����}$ CH3CH��OH��COOC2H5+H2O��
��4��������ֻ���Ȼ���̼���Ʒ�Ӧ���������ƺͶ�����̼��ˮ����Ӧ����ʽΪ
2CH3CH��OH��COOH+Na2CO3��2CH3CH��OH��COONa+H2O+CO2����
�ʴ�Ϊ��2CH3CH��OH��COOH+Na2CO3��2CH3CH��OH��COONa+H2O+CO2����
���� ���⿼���л���ṹ�����ʣ�Ϊ��Ƶ���㣬���չ����ż������ʹ�ϵ�ǽⱾ��ؼ���ע���ǻ����Ȼ����ܺ��Ʒ�Ӧ����ֻ���Ȼ��ܺ�̼���Ʒ�Ӧ��Ϊ�״��㣮

 ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�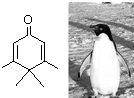
| A�� | Penguinone��һ�ַ����� | |
| B�� | Penguinone�ķ���ʽΪC9H12O | |
| C�� | Penguinone��һ��ȡ��������4�� | |
| D�� | 1molPenguinone�������3 mol H2�����ӳɷ�Ӧ |
| A�� | ��ȡ�屽������м����ˮ������ϼ��� | |
| B�� | ʵ������ȡ���������ȼ���Ũ���ᣬ�ټӱ���������Ũ���� | |
| C�� | ����ϩ�ͱ�����ϩ�ͱ��зֱ��������KMnO4��Һ�����۲��Ƿ���ɫ | |
| D�� | ��ȥ�����е�Ca2+��Mg2+��SO42-�����μ�������ʿ�����H2O��Ba��OH��2��HCl��Na2CO3 |
| A�� | Na2O | B�� | MgCl2 | C�� | CH3COOH | D�� | NaOH |
| A�� | ���������������������� | B�� | ֻ���������������� | ||
| C�� | ���������ж��� | D�� | ���ж� |
| A�� | ����������Һ��ϡ������ | |
| B�� | ���������������Ȼ�茶����Ͻ��� | |
| C�� | �Ҵ�ȼ�� | |
| D�� | ���ȷ�Ӧ |
��������
| A�� | ���� | B�� | �� | C�� | ��ϩ | D�� | �Ҵ� |
| A�� | ��ѧƽ�ⳣ����ӳ����һ���¶��¿��淴Ӧ���ܽ��е��� | |
| B�� | ����ƽ�ⳣ�������¶ȵĺ������¶���������ֵ������ | |
| C�� | һ�����淴Ӧ�Ļ�ѧƽ�ⳣ���Ͳ��뷴Ӧ��ÿ�����ʵĻ�ѧ���������й�ϵ | |
| D�� | ��ˮ�м���ǿ���ǿ�ˮ�����ӻ����������С |
| A�� | ������Һ�У�K+��Mg2+��S2-��ClO- | |
| B�� | �����£�pH=1����Һ�У�Na+��Fe3+��NO3-��SO42- | |
| C�� | �������������ݲ�������Һ�У�Na+��NH4+��Fe2+��NO3- | |
| D�� | ��AlCl3��Һ�У�K+��Na+��HCO3-��SO42- |