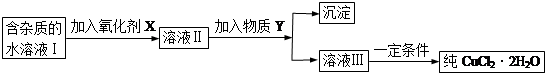��Ŀ����
�ҹ��ӰĴ����ǽ��ڵ�ij��¯��������A��ʾ���ijɷ����£�������������
| C | Si | Mn | P | S |
| 4.070% | 2.900% | 0.495% | 0.165% | 0.010% |
��2���ʵķ�Ч���൱��P2O5��������ʾ������ʱ��PԪ���γ�¯��Ca3(PO4)2����������Ũ���ᷴӦ�Ƶ���ͨ������ƣۼ��ոƣ��ɷ���CaSO4��Ca(H2PO4)2�������������ʣݡ���ij�ո���Ca(H2PO4)2����������Ϊ45.25%����P2O5���������������� %��
��3���̵���������Ϊ30%�ĸ��̸��кܺõ����ܣ����������֡���Aұ���ɺ�̼0.4%������30%�ĸ��̸֣���������ģ����������ѳ�ȥ���ɲ����̣�����100��A���Ƶø��̸� �֡�
��4��ij���ֳ����̳��ɷֺ������������±���
| | FeO | Fe2O3 | CaO |
| �̳�����ǰ��%�� | 86.40 | 4.00 | 9.60 |
Ϊ�˼����̳����ŷţ����̳���CO��Ϻ��ڿ��������գ��õ������������CaO���ս������CaO����������Ϊ8.92%������Ԫ������ģ����ս�������CaO����������������ֻ������������ɣ����������������ɺ����ʵ���֮����Σ�
��1��Mn3O4��2�֣�
��2�� 27.46
��3��132.701��3�֣�
��4����Ӧ����������� n(Fe):n(O)=1��1.4
������FeO��Fe2O3�Ļ��������ǵ����ʵ���֮��Ϊ1��2��
������Fe3O4��Fe2O3�Ļ��������ǵ����ʵ���֮��Ϊ1��1��
���������������1��100.000g A����Mn������=100.000g��0.495%=0.495g������MnxOY��OԪ�ص�����=0.687g-0.495g=0.192g����x��y="0.495/55" :0.192/16=3��4���ʸ�������Ļ�ѧʽΪMn3O4���ʴ�Ϊ��Mn3O4��
��2����Ca��H2PO4��2��дCaH4O3?P2O5����P2O5�����������T45.25%��142/234=27.46%���ո��л����ܻ���Ca3��PO4��2��������P2O5��ʾ���������������ܴ���27.46%����P2O5������������27.46%���ʴ�Ϊ����27.46%��
��3����������ɸ��̸�y�֣�����FeԪ���������䣬��y����1-30%-0.4%��=100����1-4.070%-2.9%-0.495%-0.165%-0.010%����ã�y=132.701���ʴ�Ϊ��132.701��
��4�������̳�����ǰ�̳�������Ϊ100g����n��FeO��=1.2mol��n��Fe2O3��=0.025mol��FeԪ�����ʵ���=1.2mol+0.05mol=1.25mol��������=1.25mol��56g/mol=70g���̳�����ǰ��FeԪ�ص��������䣮���պ�CaO������Ҳ���䣬���պ����ʵ�����=100*9.6%/8.92%=107.6g������OԪ�ص�����=107.6g����1-8.92%��-70g=28.0g��n��O��=28/16=1.75mol����Ӧ����������� n��Fe����n��O��=1.25mol��1.75mol=1��1.4�����̳����պ������������ƽ�����ΪFeO1.4�����ij�����������FeO��Fe2O3��Fe3O4��������FeO��Fe2O3�Ļ�����FeO��Fe2O3�ķֱ�Ϊxmol��ymol����(X+3Y)/(X+2Y)=1.4��������x��y=1��2����FeO��Fe2O3�����ʵ���֮��Ϊ1��2��������Fe3O4��Fe2O3�Ļ�����Fe3O4��Fe2O3��xmol��ymol����(4X+3Y)/(3X+2Y)=1.4��������x��y=1��1����Fe3O4��Fe2O3�����ʵ���֮��Ϊ1��1��������FeO��Fe3O4�Ļ���������ƽ����ɣ���������������FeO��Fe2O3��Fe3O4�Ļ������ü����жϣ���FeO��Fe2O3�Ļ���Fe2O3�ĺ������Ϊ2/3=66.7%����ΪFe3O4��Fe2O3�Ļ���Fe2O3�ĺ������Ϊ1/2=50%����50%��Fe2O3�����ʵ���������66.7%���𣺢�����FeO��Fe2O3�Ļ��������ǵ����ʵ���֮��Ϊ1��2��������Fe3O4��Fe2O3�Ļ��������ǵ����ʵ���֮��Ϊ1��1��������FeO��Fe3O4�Ļ�����������������FeO��Fe2O3��Fe3O4�Ļ���50%��Fe2O3�����ʵ���������66.7%��
���㣺����������Ϊ���壬�������ʵ������ؼ��㼰ƽ��ֵ����Ӧ�ã���Ŀ�ѶȽϴ��Ƕ�ѧ���ۺ������Ŀ��顣

 ÿ�α���ϵ�д�
ÿ�α���ϵ�д��ú���Al2O3������Fe2O3��SiO2���������Ʊ���ˮ������Һ��ۺ����������������������£����ֲ����������ԣ���
I�����������м������H2SO4���ȡ����衢���ˡ�
II������Һ�м���һ������FeSO4��7H2O��˫��ˮ��
III������Һ�м���Ca(OH)2���壬������Һ��pHԼΪ1�����ˡ�
IV�������ȶ��������ȣ��õ���Ʒ��
��1��Fe2O3��H2SO4��Ӧ�����ӷ���ʽ��___________��
��2������I�й��˵õ��������ɷ���________���ѧʽ����
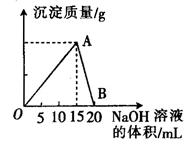
��3������I ��H2SO4��Ũ���뷴Ӧ�¶Ȼ�Ӱ���������Ľ����ʡ�������ͼ����������I ��H2SO4Ũ�ȵ����˷�Χ��__________����Ӧ�������¶���_________��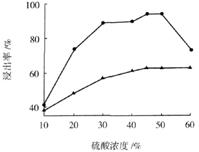

��4������II������n(Fe3+)�����ӷ���ʽ��_________��
��5������III�õ���ʽ��������[AlFe(OH)n(SO4)m]����Һ������II��Ӧ����n(Fe3+)��
n(Al3+)�sn(Fe3+)= ��
��6���о�������Һ��ۺ����������Ĵ���Խ�ߣ���ˮЧ��Խ�á���֪��
һЩ������20��ʱ���ܽ��
| ���� | Ca(OH)2 | CaSO4 | Na2SO4 |
| �ܽ��/g | 0.153 | 0.258 | 19.5 |
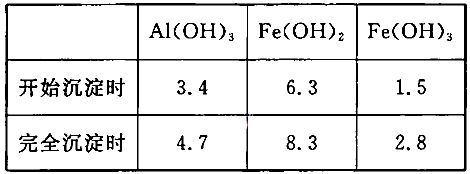
��ϱ������ݣ����Ͳ���III��ʹ��Ca(OH)2������NaOH��ԭ��__________��
��7��������Ҳ������ұ������Al���Խ���Al��������ϡ���������Һ��ͨ������ʹ����Al�ı����������ܼ�Ӳ������Ĥ����缫��Ӧʽ��_________��
 xFe2O3�������Ʊ�Al2(S04)3
xFe2O3�������Ʊ�Al2(S04)3
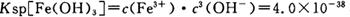 ��pH=2ʱ��Fe3����ʼ������Ũ��Ϊ_______________��
��pH=2ʱ��Fe3����ʼ������Ũ��Ϊ_______________��