��Ŀ����
���ҵĻ������÷����ܶ࣬���ú���Al2O3��SiO2������FeO xFe2O3�������Ʊ�Al2(S04)3
xFe2O3�������Ʊ�Al2(S04)3 18H2O�������������£�
18H2O�������������£�
��ش��������⣺
��1���������ϡH2SO4�ܽ�Al2O3�����ӷ���ʽ��______________��
��2�������м��˵�KMnO4Ҳ����H2O2���棬����H2O2������Ӧ�Ļ�ѧ����ʽΪ_______________��
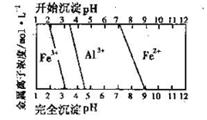
��3����֪��Ũ�Ⱦ�ΪO.1mol/L�Ľ��������ӣ������������������pH���±���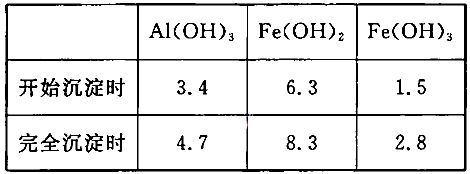
����۵�Ŀ����__________________________________________________________�����ڸ�Ũ���³�ȥ���Ļ��������pH�����Χ��___________��
��4����֪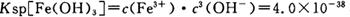 ��pH=2ʱ��Fe3����ʼ������Ũ��Ϊ_______________��
��pH=2ʱ��Fe3����ʼ������Ũ��Ϊ_______________��
��5�������ܷ�����Ӧ�����ӷ���ʽΪ__________________________________________��Ϊ����֤�ò������ù�����ȷʵ����MnO2����ѡ�õ��Լ���_________��_________��
��6�������ݡ�һϵ�в���"�����������в����õ���___________������ţ���
| A�������� | B������ | C�������� | D���ƾ���E��©�� |
��1��6H+ + Al2O3=2Al3+ + 3H2O ��2��H2O2 + 2FeSO4 + H2SO4 =" Fe2(SO4)3" + 2H2O ����3���������������������������ӣ���ͨ������PHֵ������������ת��Ϊ��������������ȥ��2.8��3.4����4��4*10-2mol/L����5��3Mn2+ + 2MnO4- + 4OH- ="5MnO2" + 2H2O ��Ũ�������������Һ����6�� B
���������������1���������ϡH2SO4�ܽ�Al2O3�����ӷ���ʽ��6H+ + Al2O3=2Al3+ + 3H2O ����2�������м��˵�KMnO4Ҳ����H2O2���棬H2O2��ǿ�����Ѷ�������������Ϊ���������ӣ�������Ӧ�Ļ�ѧ����ʽΪH2O2 + 2FeSO4 + H2SO4 =" Fe2(SO4)3" + 2H2O ����3����������ͼ��֪�ǽ������������������������ӣ���ͨ������PHֵ������������ת��Ϊ��������������ȥ��ȷ�������Ӳ�Ҫ�����������ʵ���pH��ΧΪ��2.8��3.4����4����֪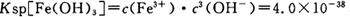 ��pH=2ʱ����c(H+)=0.01Mmol/L��c(OH-)=10-12Mmol/L������
��pH=2ʱ����c(H+)=0.01Mmol/L��c(OH-)=10-12Mmol/L������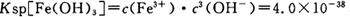 ��ʽ���ɵ�Fe3����ʼ������Ũ��Ϊ4*10-2mol/L����5�����ݲ����г��ֵ�������ɫ��ʧ��˵������������Ӳμ��˷�Ӧ���ܷ�����Ӧ�����ӷ���ʽΪ3Mn2+ + 2MnO4- + 4OH- ="5MnO2" + 2H2O ��Ϊ����֤�ò������ù�����ȷʵ����MnO2����ѡ�õ��Լ���_Ũ���Ტ���Ȼ����������Һ�����ݲ�������˵������6�������ݡ�һϵ�в���"���У�����Ũ�������½ᾧ�����ˣ������õ��� B��������
��ʽ���ɵ�Fe3����ʼ������Ũ��Ϊ4*10-2mol/L����5�����ݲ����г��ֵ�������ɫ��ʧ��˵������������Ӳμ��˷�Ӧ���ܷ�����Ӧ�����ӷ���ʽΪ3Mn2+ + 2MnO4- + 4OH- ="5MnO2" + 2H2O ��Ϊ����֤�ò������ù�����ȷʵ����MnO2����ѡ�õ��Լ���_Ũ���Ტ���Ȼ����������Һ�����ݲ�������˵������6�������ݡ�һϵ�в���"���У�����Ũ�������½ᾧ�����ˣ������õ��� B��������
���㣺���⿼�����ӷ���ʽ����д��ʵ��ԭ���ķ����ͳ����ܽ�ƽ����ؼ��㡣

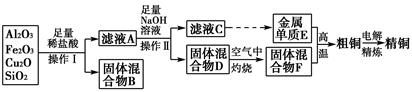
�������㷺�����л��ϳɡ�ӡȾ��ҵ�ȡ���ҵ��������Ϊԭ�ϣ���Ҫ�ɷ�ΪAl��������Al2O3��Fe2O3��SiO2��CaO��MgO�ȣ��Ʊ��������Ĺ����������£�
��֪��Al��OH��3�������ܽ��pH���±���
| Al��OH��3 | ��ʼ���� | ������ȫ | ������ʼ�ܽ� | �����ܽ���ȫ |
| pH | 3.3 | 5.0 | 7.8 | 12.8 |
�ش��������⣺
��1������ʱ����������Ӧ�����ӷ���ʽΪ________________________________��
��2�����������е�һ�μ��������pH��7.0��Ŀ����__________________________________________________________��
pH��7.0ʱ����Һ��c��Al3������________��ͨ�������£�Ksp[Al��OH��3]��1.3��10��33����
��3�����������ʸ��ᡢ��ķ�Ӧ�����ڼ��ܡ���pH��7.0�����ܹ����У����������ļ������ʵ���֮��n1��NaOH����n2��HNO3����n3��HNO3����________��
��4������1 t����������������[Al��NO3��3��9H2O]�����������7.5 t���������壬��������������Ԫ�ص���ʧ��Ϊ10%��������������Ԫ�ص�����������
�ҹ��ӰĴ����ǽ��ڵ�ij��¯��������A��ʾ���ijɷ����£�������������
| C | Si | Mn | P | S |
| 4.070% | 2.900% | 0.495% | 0.165% | 0.010% |
��2���ʵķ�Ч���൱��P2O5��������ʾ������ʱ��PԪ���γ�¯��Ca3(PO4)2����������Ũ���ᷴӦ�Ƶ���ͨ������ƣۼ��ոƣ��ɷ���CaSO4��Ca(H2PO4)2�������������ʣݡ���ij�ո���Ca(H2PO4)2����������Ϊ45.25%����P2O5���������������� %��
��3���̵���������Ϊ30%�ĸ��̸��кܺõ����ܣ����������֡���Aұ���ɺ�̼0.4%������30%�ĸ��̸֣���������ģ����������ѳ�ȥ���ɲ����̣�����100��A���Ƶø��̸� �֡�
��4��ij���ֳ����̳��ɷֺ������������±���
| | FeO | Fe2O3 | CaO |
| �̳�����ǰ��%�� | 86.40 | 4.00 | 9.60 |
Ϊ�˼����̳����ŷţ����̳���CO��Ϻ��ڿ��������գ��õ������������CaO���ս������CaO����������Ϊ8.92%������Ԫ������ģ����ս�������CaO����������������ֻ������������ɣ����������������ɺ����ʵ���֮����Σ�


 FeCO3(s)��H2CO3(aq)��ƽ�ⳣ��Ϊ_______��
FeCO3(s)��H2CO3(aq)��ƽ�ⳣ��Ϊ_______��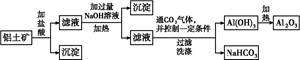

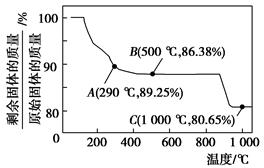
 C�� ��
C�� ��