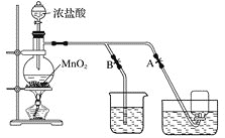
��Ŀ����
����Ŀ��ij��ѧ��ȤС��Ϊ̽��SO2�����ʣ�����ͼ��ʾװ�ý���ʵ�顣
��֪��Na2SO3+H2SO4��Na2SO4+SO2��+H2O

�ش��������⣺
��1��װ��A��ʢ��Ũ���������������____________��
��2��װ��B��Ŀ����̽��SO2��Ʒ�����õĿ����ԣ���д��ʵ�����������____________��
��3��װ��C�б�����SO2��____________�ԣ�װ��D�б�����SO2��____________�ԣ�װ��D�з�����Ӧ�Ļ�ѧ����ʽΪ____________��
��4��Fװ�õ�������____________��©����������____________��
��5��E�в�����ɫ�������ð�ɫ�����Ļ�ѧ�ɷ�Ϊ____________�����ţ���ͬ��,���ʵ��֤������ж�____________��
A.BaSO3 B.BaSO4 C.BaSO3��BaSO4
��6������úȼ�ղ�����������ֱ���ŷŵ������У���������Ҫ����������____________��
A.����ЧӦ B.���� C.�۳���Ⱦ D.ˮ�帻Ӫ����
��ҵ��Ϊʵ��ȼú����ͨ������ʯ��ʯ�õ���ʯ�ң�����ʯ��Ϊ���������������SO2��Ӧ�Ӷ�����̶������������������ϡ�д�����н���̶��Ļ�ѧ����ʽ��____________��
���𰸡���Һ©�� ͨSO2Ʒ������ɫ���رշ�Һ©������ֹͣͨSO2������Ʒ����Һ����Һ�ָ���ԭ������ɫ ��ԭ���ԣ� �������ԣ� 2H2S+SO2=3S��+2H2O ����δ��Ӧ���SO2 ������ B ���ð�ɫ��������ϡ���ᣬ�������ܽ� ABC 2CaO+2SO2+O2=2CaSO4
��������
��ʵ��Ŀ��̽��SO2�����ʣ�����ͨ��Aװ���Ʊ�SO2��������SO2ͨ��Ʒ����Һ��Ʒ����ɫ֤��SO2����Ư���ԣ���Ϊ����Ư�ײ��ȶ����رշ�Һ©���Ļ���ֹͣͨ�����壬����ʢ����ɫ��Ʒ���Թܣ�Ʒ��ָ�ԭ������ɫ��֤����Ư���ǻ���Ư�ף����ȶ���ͨ�����Ը�����أ���ɫ��ɫ���������·�Ӧ��5SO2+2MnO4��+2H2O=2Mn2++5SO42��+ 4H+��֤��SO2���л�ԭ�ԡ�ͨ��H2S��Һ�����ֵ���ɫ���ǣ�2H2S+SO2=3S��+2H2O���÷�Ӧ����SO2���������ԡ�ͨ�����ᱵˮ��Һ�����������ˮ��Һ�����ԣ�NO3����H+������ǿ�����ԣ���������SO2�Ӷ�������ɫ�������ᱵ������õ��õ�©����ֹ������������������Һ����β�����ա�
��1��װ��A��ʢ��Ũ��������������Ƿ�Һ©����
��2��������SO2ͨ��Ʒ����Һ��Ʒ����ɫ֤��SO2����Ư���ԣ��رշ�Һ©���Ļ���ֹͣͨ�����壬����ʢ����ɫ��Ʒ���Թܣ�Ʒ��ָ�ԭ������ɫ��֤����Ư���ǻ���Ư�ף����ȶ���
��3�����ݷ�����װ��C�б�����SO2�Ļ�ԭ�ԣ�װ��D�б�����SO2�������ԣ�װ��D������Ӧ�Ļ�ѧ����ʽΪ2H2S+SO2=3S��+2H2O��
��4�����ݷ���Fװ�õ�����������δ��Ӧ���SO2��©���������Ƿ�������
��5�����ݷ�����E������ɫ����BaSO4���������ʵ�飺���ð�ɫ��������ϡ���ᣬ�������ܽ⣬������ʵ������֤��ֻ��BaSO4����BaSO3��
��6������úȼ�ղ����������к��д����Ķ�����̼����������ͷ۳�����������Ҫ��������������ЧӦ�����ꡢ�۳���Ⱦ��ˮ�帻Ӫ������������ˮ����ҵ��ˮ�ȵ��ŷŵ��µ���Ԫ�س��꣬��ѡABC������ʹ�õ��͵ĸɷ���������ѧ��Ӧԭ��Ϊ��2CaO+2SO2+O2= 2CaSO4��

����Ŀ����������ʵ��������������ó��Ľ�����ȷ����
ѡ�� | ʵ����������� | ���� |
A | ����Һ�еμ�Na2CO3��Һ����Һ���� | ���ӵ�����ǿ��H2CO3������ |
B | ����������������Ƶ��Ҵ���Һ���Ⱥ����������ͨ��������Ȼ�̼��Һ����Һ��ɫ | �����鷢����ȥ��Ӧ |
C |
|
|
D | �� |
|
A.AB.BC.CD.D