��Ŀ����
����Ŀ��������ĿҪ���û�ѧ���Իش����⡣
��I����ȥ���������ڵ�����ͨ�����õ�ʵ�鷽����ʲô���������ں����ϡ�
(1)CH3CH2OH(H2O)_____________________________________________________��
(2)![]() (NaCl)____________________________________________��
(NaCl)____________________________________________��
(3) ![]() (Br2)___________________________________________________��
(Br2)___________________________________________________��
��II��ij�л����ʵ��ʽΪC2H6O���������Dzⶨ����Է������������ⶨ�õ���ͼ1��ʾ������ͼ������ú˴Ź����Ǵ������л���õ���ͼ2��ʾ�ĺ˴Ź�������ͼ��

�Իش��������⣺
(1)���л����������Է�������Ϊ________��
(2)��д�����л�������Ľṹ��ʽ________��
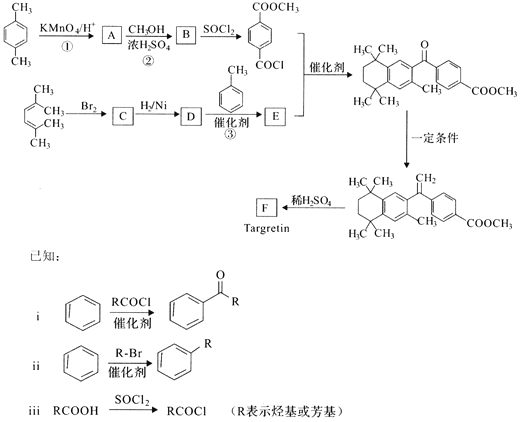
��III���л���E(C3H3Cl3)��һ�ֲ�ǰ���ݼ���ǰ�壬��ϳ�·�����¡�

��֪D�ڷ�Ӧ���������ɵ�E����ṹֻ��һ�ֿ��ܣ�E��������3�ֲ�ͬ���͵���(�����ǿռ��칹)���Իش��������⣺
(1)��������е���Ϣ�Ʋ���A�Ľṹ��ʽΪ______________����A��ͬϵ���У���Է���������С���������Ӿ۷�Ӧ�Ļ�ѧ����ʽΪ____________________________��
(2)д�����з�Ӧ�����ͣ���Ӧ����____________����Ӧ����________��
(3)��������е���Ϣ�Ʋ��л���D��������______________��
(4)��д����Ӧ�۵Ļ�ѧ����ʽ��____________________________________��
���𰸡� ����(�ɼ�����ʯ��) �ؽᾧ ��ϴ��Һ(���NaOH��Һ����Һ) 46 CH3OCH3 CH2=CH-CH3 nCH2=CH2![]() -[CH2-CH2]-n ȡ����Ӧ ��ȥ��Ӧ �������10��1,2,2,3-���ȱ��� CH2ClCHClCH2Cl��NaOH
-[CH2-CH2]-n ȡ����Ӧ ��ȥ��Ӧ �������10��1,2,2,3-���ȱ��� CH2ClCHClCH2Cl��NaOH![]() CH2=CCl��CH2Cl��NaCl��H2O
CH2=CCl��CH2Cl��NaCl��H2O
��������������Ҫ�����л���Ľṹ�����ʡ�
��I��(1)CH3CH2OH��H2O���ܣ�����ѡ������(�ɼ�����ʯ��)��
(2)![]() ����ˮ��NaCl������ˮ�����ǵ��ܽ�Ȳ�������ѡ���ؽᾧ��
����ˮ��NaCl������ˮ�����ǵ��ܽ�Ȳ�������ѡ���ؽᾧ��
(3) ��NaOH��Һ��ȥBr2��![]() ������ˮ���ٷ�Һ������ѡ�ü�Һ��Һ��
������ˮ���ٷ�Һ������ѡ�ü�Һ��Һ��
��II��(1)������ͼ���ɸ��л��������ʵ��ʽC2H6O�����Եõ�����Է�������Ϊ46��
(2) ���л�������ֻ��һ����ԭ�ӣ����Ը��л��ﲻ���Ҵ����Ǽ��ѣ���ṹ��ʽΪCH3OCH3��
��III��(1) B����1,2,3�����ȱ����Ǽӳɷ�Ӧ����Ӧ����ȡ����Ӧ���Ʋ���A�Ľṹ��ʽΪCH2=CH-CH3����A��ͬϵ���У���Է���������С������ϩ�����Ӿ۷�Ӧ�Ļ�ѧ����ʽΪnCH2=CH2![]() -[CH2-CH2]- n��
-[CH2-CH2]- n��
(2)���з�Ӧ�����ͣ�B����1,2,3�����ȱ����Ǽӳɷ�Ӧ�����Է�Ӧ����ȡ����Ӧ���ӷ�Ӧ������֪1,2,3�����ȱ��鷢��±��������ȥ��Ӧ�����Է�Ӧ������ȥ��Ӧ��
(3)����D�ڷ�Ӧ���������ɵ�E�Ľṹֻ��һ�ֿ��ܣ�E��������3�ֲ�ͬ���͵������Ʋ��л���D��������1,2,2,3-���ȱ�����
(4)��Ӧ�۵Ļ�ѧ����ʽ��CH2ClCHClCH2Cl��NaOH![]() CH2=CCl��CH2Cl��NaCl��H2O��
CH2=CCl��CH2Cl��NaCl��H2O��

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д� �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�����Ŀ��A��B��C��D��E�� F��G�����ڱ��ж����ڵ�����Ԫ�أ��й����ʻ�ṹ��Ϣ���±���
Ԫ�� | �й����ʻ�ṹ��Ϣ |
A | �ؿ��к�������Ԫ�� |
B | B��������A�����ӵ�������ͬ���������������е������Ӱ뾶��С�� |
C | C��Bͬ���ڣ���������������ԭ�Ӱ뾶����(ϡ���������) |
D | Dԭ�������������ǵ��Ӳ�����2�������⻯���г�������ζ |
E | E��Dͬ���ڣ����ڸ�������ԭ�Ӱ뾶��С |
F | F���⻯�������������ˮ���ﷴӦ����һ�����ӻ����� |
G | G���γɻ�������������Ԫ�� |
(1) BԪ�ط���Ϊ____��A��C��ԭ�Ӹ�����Ϊ1��1�γɵĻ�����ĵ���ʽΪ___���õ���ʽ��ʾC��E�γɻ�����Ĺ���____�� D�γɵļ����ӵĽṹʾ��ͼΪ____��
(2) F���⻯������____(���Ի�Ǽ���)���γɵķ��ӣ�д��ʵ�����Ʊ����⻯��Ļ�ѧ����ʽ____��
(3) �ǽ�����D____E(����ڻ�С��)�����ԭ�ӽṹ�ĽǶȽ���ԭ��__��