��Ŀ����
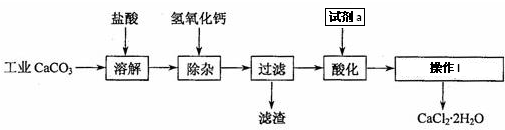
14������Ǧ�׳Ƹ��ƣ�������ˮ���㷺����Ϳ�ϡ���ī��������Ϻ��Ľ���Ʒ�ȹ�ҵ��ʵ����ģ�ҵ���ø����ࣨ����Cr2O3��Fe2O3��Al2O3��SiO2�ȣ��Ʊ����ƵĹ����������£�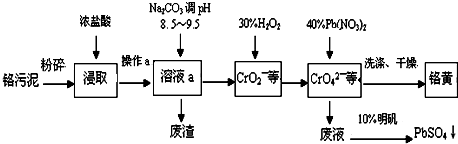
��1��������������Ŀ��������Ӵ��������߽�ȡ�ʣ�
��2����������Ҫ�ɷ���Al��OH��3��Fe��OH��3����֪25��ʱ��Al��OH��3��Ksp=1.3��10-33������¶��·�ӦAl3++3H2O?Al��OH��3+3H+��ƽ�ⳣ��Ϊ7.7��10-10��
��3��д������30%H2O2�����з��������ӷ�Ӧ����ʽ��3H2O2+2CrO2-+2OH-=2CrO42-+4H2O��
��4������Pb��NO3��2����CrO42-ʱ����������Ƿ���ȫ�ķ����Ǿ��÷ֲ��ȡ�ϲ���Һ�������μ�Pb��NO3��2��Һ���������ɣ�˵��������ȫ��
���� ���̷�����֪�������ࣨ��Ҫ�ɷ�ΪCr2O3������ΪFe2O3��Al2O3��SiO2��Ϊԭ�Ϸ�����ڷ�Ӧ���м���Ũ�����ȡ����������費��Ӧ�������˿ɵõ�����Ϊ�������裬��Һa�к���Fe3+��Al3+��Cr3+�ȣ�����̼������Һ����pH8.5��9.5���ɳ�ȥFe3+��Al3+���õ��ķ���ΪAl��OH��3��Fe��OH��3����CrO22-��Һ�м���������⣬�������ɵõ�CrO42-��Һ��Ȼ�����Pb��NO3��2��Һ���ɵõ�PbCrO4���������˺��Һ�к���Pb��NO3��2�����������ɵõ�����Ǧ��
��1��������������������������ĽӴ��������������߽�ȡ�ʣ�
��2��Ksp[Al��OH��3]=c��Al3+��•c3��OH-����ˮ��ƽ�ⳣ��Kh=$\frac{{c}^{3}��{H}^{+}��}{c��A{l}^{3+}��}$����Ksp[Al��OH��3]•Kh=��kw��3���ݴ˼��㣻
��3��������ͼ��֪������30%H2O2��Ŀ�����ڼ��������½�CrO2-����ΪCrO42-��ͬʱ����ˮ��
��4����������Ƿ���ȫ�ķ����ǣ����÷ֲ��ȡ�ϲ���Һ�������μ�Pb��NO3��2��Һ���������ɣ�˵��������ȫ��
��� �⣺���̷�����֪�������ࣨ��Ҫ�ɷ�ΪCr2O3������ΪFe2O3��Al2O3��SiO2��Ϊԭ�Ϸ�����ڷ�Ӧ���м���Ũ�����ȡ����������費��Ӧ�������˿ɵõ�����Ϊ�������裬��Һa�к���Fe3+��Al3+��Cr3+�ȣ�����̼������Һ����pH8.5��9.5���ɳ�ȥFe3+��Al3+���õ��ķ���ΪAl��OH��3��Fe��OH��3����CrO22-��Һ�м���������⣬�������ɵõ�CrO42-��Һ��Ȼ�����Pb��NO3��2��Һ���ɵõ�PbCrO4���������˺��Һ�к���Pb��NO3��2�����������ɵõ�����Ǧ��
��1��������������������������ĽӴ��������������߽�ȡ�ʣ��ʴ�Ϊ������Ӵ��������߽�ȡ�ʣ�
��2��Ksp[Al��OH��3]=c��Al3+��•c3��OH-��=1.3��10-33��ˮ��ƽ�ⳣ��Kh=$\frac{{c}^{3}��{H}^{+}��}{c��A{l}^{3+}��}$����Ksp[Al��OH��3]•Kh=��kw��3=��10-14��3=10-42������Kh=$\frac{1{0}^{-42}}{1.3��1{0}^{-33}}$=7.7��10-10���ʴ�Ϊ��7.7��10-10��
��3��������ͼ��֪������30%H2O2��Ŀ�����ڼ��������½�CrO2-����ΪCrO42-��ͬʱ����ˮ����Ӧ���ӷ���ʽΪ3H2O2+2CrO2-+2OH-=2CrO42-+4H2O���ʴ�Ϊ��3H2O2+2CrO2-+2OH-=2CrO42-+4H2O��
��4����������Ƿ���ȫ�ķ����ǣ����÷ֲ��ȡ�ϲ���Һ�������μ�Pb��NO3��2��Һ���������ɣ�˵��������ȫ���ʴ�Ϊ�����÷ֲ��ȡ�ϲ���Һ�������μ�Pb��NO3��2��Һ���������ɣ�˵��������ȫ��
���� ���⿼�����ʵķ��롢�ᴿ���ۺ�Ӧ�ã�Ϊ�߿��������ͣ�������ѧ���ķ���������ʵ�������Ŀ��飬����ע��ƽ�ⳣ���ļ��㡢���ӵļ��顢������ԭ��Ӧ��֪ʶ�㣬�ѶȲ������ӵļ����Ǹ߿����ȵ㣬ע�⣨4�����л�ѧ���Ե���ȷ���ã�Ϊ�״��㣮

| A�� | ��ʹƷ����Һ��ɫ | B�� | ����NaOH��Һ��Ӧ | ||
| C�� | ����H2O��Ӧ����H2SO4 | D�� | һ������������O2��Ӧ����SO3 |

�����Ϣ���£������Ȼ�����ˮ����ˮ��
���������������ڸ����¾���������ֱ�ӷ�Ӧ������Ӧ���Ȼ���
���й����ʵ������������±���
| ���� | SiCl4 | BCl3 | AlCl3 | FeCl3 | PCl5 |
| �е�/�� | -57.7 | 12.8 | - | 315 | - |
| �۵�/�� | -70.0 | -107.2 | - | - | - |
| �����¶�/�� | - | - | 180 | 300 | 162 |
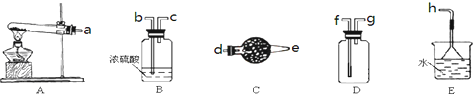
��2��װ��A��g�ܵ�������ƽ��ѹǿ��ʹҺ��˳����������ֹ©����
��3��װ��E��hƿ�ռ����Ĵֲ����ͨ���������ƶ�����õ��ߴ������Ȼ��裬�����IJ������У�����Ԫ������ܻ����е�����Ԫ����Al��P����дԪ�ط��ţ���
��4��������f��װ�â��ҷ�����f��װ�â���װ�â��в���֮�����������һ�����������������ֱ����װ�â��i���Ӹ����j
��5��Ϊ�˷�������������Ԫ�صĺ������Ƚ�������Ԥ����������Ԫ�ػ�ԭ��Fe2+������KMnO4����Һ�����������½���������ԭ�ζ�����Ӧ�����ӷ���ʽ�ǣ�5Fe2++MnO${\;}_{4}^{-}$+8H+=5Fe3++Mn2++4H2O ijͬѧ��ȡ5.000g�����Ԥ������������ƿ�����Ƴ�100ml��Һ����ȡ25.00ml��������Һ����1.000��10-2mol•L-1KMnO4����Һ�ζ����ﵽ�ζ��յ�ʱ�����ı���Һ20.00ml�������������Ԫ�ص�����������4.480%����д��������̣�
 ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ
ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ��֪��
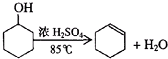
| �ܶȣ�g/cm3�� | �۵� ���棩 | �е� ���棩 | �ܽ��� | |
| ���Ѵ� | 0.96 | 25 | 161 | ������ˮ |
| ����ϩ | 0.81 | -103 | 83 | ������ˮ |
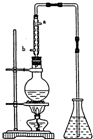
��12.5mL�����������Թ�A�У��ټ���lmLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ�������Ƿ�ֹ���У�����B���˵�������е�����������������
���Թ�C���ڱ�ˮԡ�е�Ŀ���ǽ�һ����ȴ����ֹ����ϩ�ӷ���
��2���Ʊ���Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȣ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ���ϲ㣨���ϻ��£�����Һ����c �������ţ�ϴ�ӣ�
a��KMnO4��Һ b��ϡH2SO4 c��Na2CO3��Һ
���ٽ�����ϩ���ɵõ���Ʒ��
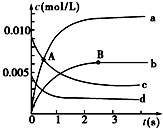 ��2L�ܱ������У�800��ʱ��Ӧ2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����
��2L�ܱ������У�800��ʱ��Ӧ2NO��g��+O2��g��?2NO2��g����ϵ�У�n��NO����ʱ��ı仯�����| ʱ�䣨s�� | 0 | 1 | 2 | 3 | 4 | 5 |
| n��NO��/mol | 0.020 | 0.010 | 0.008 | 0.007 | 0.007 | 0.007 |
��2��ͼ�б�ʾO2�仯��������d����NO2��ʾ��0��2s�ڸ÷�Ӧ��ƽ������v=3.0��10-3mol•L-1•s-1��
��3����˵���÷�Ӧ�Ѿ��ﵽƽ��״̬����ad��
a��������ѹǿ���ֲ��䡡��������b��v��NO��=2v��O2��
c�������ڵ��ܶȱ��ֲ���d��v����NO2��=2v����O2��
��4����ʹ�÷�Ӧ�ķ�Ӧ�����������acd��
a���ʵ������¶� b����ʱ�����NO2����c������O2��Ũ�� d��ѡ���Ч�Ĵ�����
 ������ȩͨ��Ϊ40%���ҵ���ȩ��Һ�����õ���ȩ��Һ������ֲ������ϲ�Ϊ��ɫ��״Һ�壬�²�Ϊˮ��Һ���ݲⶨ���ϲ�����Ϊ��ȩ�ļӺ��C2H4O��n�����ķе��ˮ�ķе�ߣ���������ȩ������ȩ����Һ���ױ�������Ϊ�ӱ��ʵ���ȩ��Һ����ȡ��ȩ���Եõ���Һ�������������·�Ӧԭ������C2H4O��n$\stackrel{{H}^{+}}{��}$ n��C2H4O��
������ȩͨ��Ϊ40%���ҵ���ȩ��Һ�����õ���ȩ��Һ������ֲ������ϲ�Ϊ��ɫ��״Һ�壬�²�Ϊˮ��Һ���ݲⶨ���ϲ�����Ϊ��ȩ�ļӺ��C2H4O��n�����ķе��ˮ�ķе�ߣ���������ȩ������ȩ����Һ���ױ�������Ϊ�ӱ��ʵ���ȩ��Һ����ȡ��ȩ���Եõ���Һ�������������·�Ӧԭ������C2H4O��n$\stackrel{{H}^{+}}{��}$ n��C2H4O�� ��
��