��Ŀ����
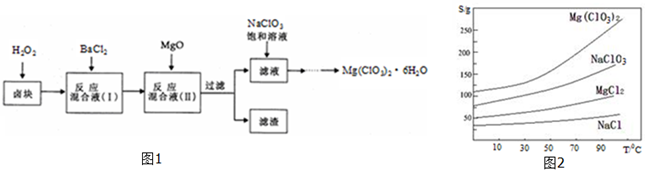
9����ŨCaCl2��Һ��ͨ��NH3��CO2�������Ƶ�����̼��ƣ�����ֱ����1��10nm֮�䣩����ͼ��ʾA��EΪʵ���ҳ���������װ�ã����̶ֹ��г�װ����ȥ���������Ҫ��ش����⣮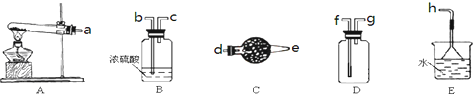
��1��ʵ�����Ʊ�NH3�ķ�Ӧ����ʽ��Ca��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��2��ʵ������ȡ���ռ������NH3����ѡ����������װ�õĽӿ�����˳���ǣ�ѡ����ĸ����a��d��e��g��f��h��
��3����ŨCaCl2��Һ��ͨ��NH3��CO2����������̼���ʱ��Ӧ��ͨ���������NH3����д��������̼��Ƶ����ӷ���ʽCa2++2NH3+H2O+CO2=CaCO3��+2NH4+��
��4����ŨCaCl2��Һ��NH3������ȷ������£�CO2�����������ᵼ������̼��Ʋ����½�����CO2������Һ�д������ڵ������У�������������ʵĵ��������ˮ��������������ӣ�Ca 2+��NH4+��HCO3-��Cl-��
��5��ȡ��Ӧ��ȥ����CaCO3����Һ�ֱ�������ʵ�飬����ʵ���жϺ������ǣ�B��
A���μ�����Na2CO3��Һ�����г���˵��CO2һ������
B���μ��������ᣬ�������ݣ�CO2һ������
C��������ҺpH��������7��CO2һ��������
D���μ�����BaCl2��Һ����������CO2һ��û�й���
��6������Ƽ�ʵ�鷽�����ж�����̼�����Ʒ�����Ƿ�Ϊ������ˮ��ϣ�����۲춡���ЧӦ��
���� ��1��ʵ������ȡ���������Ȼ�狀��������Ʒ�Ӧ�Ƶã������Ȼ��ơ�������ˮ��
��2�����ݷ�Ӧ���״̬����Ӧ����ѡȡ��Ӧװ�ã�����������ܽ��ԡ��ܶ�ѡ���ռ�װ�ã�ע�����ܵ�ʹ��ԭ���Ǵ�ڽ�С�ڳ���
��3�����ݶ�����̼�Ͱ������ܽ����ж���ͨ������壬�ɷ�Ӧ���������д������ʽ��
��4����ŨCaCl2��Һ��NH3������ȷ������£���������̼������������̼��̼��ƺ�ˮ��Ӧ����̼����ƣ�
��5��ȡ��Ӧ��ȥ����CaCO3����Һ���μ�����Na2CO3��Һ�����г�������˵��CO2���㣬������̼������Һ��Ҳ���и����ӣ�
�μ��������ᣬ�������ݣ�Ϊ̼��������Ӻ���ķ�Ӧ��CO2һ��������������ҺpH��������7��˵����Һ�ʼ��ԣ�����Ϊ̼��������Һ���μ�����BaCl2��Һ��������������Ϊ̼���Ⱶ��Һ��
��6��̼�����Ʒ�������Ϊ�����������Ŀ����ڽ��巶Χ�ڣ����Ը��ݽ��������ȷ��ʵ�鷽����
��� �⣺��1��ʵ�������Ȼ�狀ͼ�ʯ�ҷ�Ӧ��ȡ�������������η�Ӧ�����¼�����Σ���ѧ����ʽΪ2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��Ca��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��2��ʵ������ȡ�������ù��塢���������װ�ã�����ӦѡAΪ��Ӧװ�ã��������к���ˮ���������ڼ������壬����Ӧѡ�������������ˮ��������ѡC��������������ˮ���Ұ������ܶ�С�ڿ������ܶȣ�����Ӧ���������ſ������ռ�����ѡD�������д̼�����ζ�����Բ���ֱ���ſգ�������������ˮ������β������Ӧ���÷�ֹ����װ�ã���ˮ���ռ��ɣ���ѡE��ע�����ܵ�ʹ��ԭ���Ǵ�ڽ�С�ڳ�����������װ�õĽӿ�����˳����d��e��g��f��
�ʴ�Ϊ��d��e��g��f��
��3��������������ˮ��������̼��������ˮ������Ӧ��ͨ�백������������ˮ���ɰ�ˮ����Һ�ʼ��ԣ�������̼���������壬�ܺͼӦ����̼��泥�̼��狀��Ȼ��Ʒ������ֽⷴӦ����̼��ƺ��Ȼ��CaCl2+CO2+2NH3+H2O=CaCO3��+2NH4Cl�����ӷ�ӦΪ��Ca2++2NH3+H2O+CO2=CaCO3��+2NH4+��
�ʴ�Ϊ��NH3��Ca2++2NH3+H2O+CO2=CaCO3��+2NH4+��
��4����������̼������������̼��̼��ƺ�ˮ��Ӧ����̼����Ƶ���̼��Ƶ������٣�̼������ǿ����Ե�ǿ����ʣ���ˮ��Һ�����������ӡ�̼��������ӣ���Һ�л����������Ӻ�笠����ӣ�
�ʴ�Ϊ��Ca 2+��NH4+��HCO3-��Cl-��
��5�����ݣ�4����֪����CO2������Һ�д������ڵ�������Ca 2+��NH4+��HCO3-��Cl-��
A���μ�����Na2CO3��Һ�����г�����Ϊ̼������Ӻ����ӵķ�Ӧ������ΪCO2��������Һ�д���HCO3-����A����
B���μ��������ᣬ�������ݣ�Ϊ̼��������Ӻ���ķ�Ӧ��CO2һ����������B��ȷ��
C��̼������Ϊ�������ʽ�Σ���Һ�ʼ��ԣ�������ҺpH��������7��Ҳ��Ϊ̼��������Һ����CO2�������������C����
D��CO2������Һ�д������ڵ�������Ca 2+��NH4+��HCO3-��Cl-���μ�����BaCl2��Һ����������D����
�ʴ�Ϊ��B��
��6��̼�����Ʒ�������Ϊ�����������Ŀ����ڽ��巶Χ�ڣ����Ծ��н�������ʣ����ý���Ķ����ЧӦ�жϣ�ȡ������Ʒ��ˮ����γɷ�ɢϵ����һ�������䣬������һ��������ͨ·�����������������ǣ�
�ʴ�Ϊ����ˮ��ϣ�����۲춡���ЧӦ��
���� ���⿼��������̼��Ƶ�ʵ�����Ʒ�����Ŀ�Ѷ��еȣ�������ѧ���ķ�����ʵ�������Ŀ��飬��ȷԭ���ǽ���ؼ���ע����ݷ�Ӧ���״̬����Ӧ����ѡȡ��Ӧװ�ã�����������ܽ��ԡ��ܶ�ѡ���ռ�װ�ã�

 ��ǰ����ϵ�д�
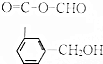
��ǰ����ϵ�д� ��������������ȡȾ�Ϻ�һЩҩ�����Ҫ�м��壬���ɱ�����Ũ���ᣨHO-SO3H���ǻ��õ���ʵ���ҿ�������ͼװ�úϳɶ���������
��������������ȡȾ�Ϻ�һЩҩ�����Ҫ�м��壬���ɱ�����Ũ���ᣨHO-SO3H���ǻ��õ���ʵ���ҿ�������ͼװ�úϳɶ���������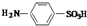
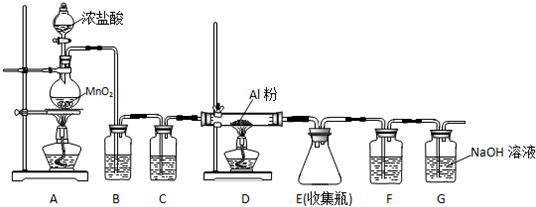
ʵ�鲽�����£�
����һ��250mL������ƿ�м���10mL��������������B������ƿ������ˮ�У������ؼ���18mLŨ���ᣮ
�ڰ�������ƿ������ԡ�л������ȣ������¶�Ϊ180�桫190�棬��Ӧ1��1.5h��
�۽���ӦҺ��ȴ��Լ50�棬����ʢ��100mL��ˮ���ձ��У������Ұ�ɫ���壬���в���C����������ˮϴ�ӹ��壬�õ�����������ֲ�Ʒ��
�ܶԴֲ�Ʒ���в���D���ٵ��º�ɼ��õ������Ķ��������ᣮ
����Ϊ�����л���IJ����������ʣ���ش��й����⣮
| ���� | ���������� | |
| �۵� | -6.3�� | 288�� |
| �е� | 184�� | ��300�濪ʼ�ֽ�̿���� |
| ˮ���� | ����ˮ | ������ˮ��������ˮ |
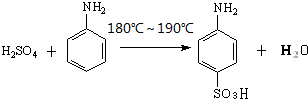 ��
����2��װ��A������Ϊ�����ܣ�������ʹ��������������
��3������ B�������Ƿ����У�����C�������ǹ��ˣ�
��4������˵����ȷ����AD
A����Һ����ȵĺô��Ƿ�Ӧ�����Ⱦ��ȣ����ڿ����¶�
B����ʵ�鲽���Ҳ���Ը���ˮԡ����
C������۽���ӦҺ����100mL��ˮ��Ŀ���Ƿ��뱽���Ͷ���������
D���������������ˮϴ�ӵ�Ŀ���dz�ȥ�������Ŀ���������ͬʱ����ϴ�ӹ����е���ʧ��
��5������D���������ؽᾧ��������̰�������������ˮ�ܽ�ֲ�Ʒ����ȴ�ᾧ�����ˡ�ϴ�ӣ�

| A�� | �Ʊ���ʽ�����������˹�������������� | |
| B�� | Ϊ��ֹNH4HCO3�ֽ⣬����FeCO3���ڽϵ��¶��½��� | |
| C�� | ����KSCN��Һ���飨NH4��2Fe��SO4��2�Ƿ����� | |
| D�� | �Ʊ���NH4��2Fe��SO4��2�����������ܽ�ȱ�FeSO4���ܽ�ȴ���һ���� |


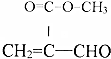
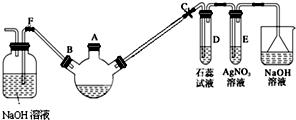 ij��ѧ������ȤС��ѧ������ͼ��ʾ��װ��̽������Һ��ķ�Ӧ���Ʊ��屽���������ش��������⣺
ij��ѧ������ȤС��ѧ������ͼ��ʾ��װ��̽������Һ��ķ�Ӧ���Ʊ��屽���������ش��������⣺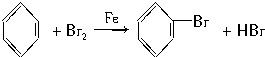 ��
��