��Ŀ����
10���±���ʾΪ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�أ�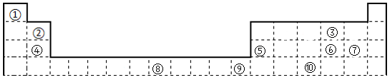
��ش��������⣺
��1����������d����Ԫ���Ǣࣨ���ţ���Ԫ�آ�ԭ�ӵļ۲���ӵĵ����Ų�ͼΪ
 ��Ԫ�آ�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ1s22s22p63s23p63d104s2��
��Ԫ�آ�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ1s22s22p63s23p63d104s2����2���ܢݢޢ�����Ԫ�صĵ�һ�������ɴ�С��˳����Cl��S��Mg��Al����Ԫ�ط��ű�ʾ����
��3��ijԪ�صļ۵����Ų�ʽΪnsnnpn+1����Ԫ����Ԫ�آ��γɵķ���X�Ŀռ乹��Ϊ�����Σ�
��4��ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ����ϱ���Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʣ���д��Ԫ�آڵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��Be��OH��2+2NaOH�TNa2BeO2+2H2O��
��5����֪Ԫ�آ��+3����������ϡ������Һ�пɱ�Ԫ�آ�ĵ��ʻ�ԭ��������̬�⻯����ﻹ�������κ�ˮ���÷�Ӧ�Ļ�ѧ����ʽ��As2O3+6Zn+6H2SO4=2AsH3��+6ZnSO4+3H2O��
���� ��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪBe����ΪO����ΪMg����ΪAl����ΪS����ΪCl����ΪFe����ΪZn����ΪAs��
��1��d��Ԫ�ذ���3-10��Ԫ�أ�����������ϵԪ�ء��ϵԪ�س��⣩����ΪO�����ڵ������ڢ�A�壬�۵����Ų�ʽΪ�۵����Ų�ʽΪ2s22p4����������������ԭ�������ع����۵����Ų�ͼ����ΪZn��ԭ�Ӻ��������Ϊ30����Ϻ�������Ų�������д��
��2��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ�����A����AԪ�ص�һ�����ܸ���ͬ��������Ԫ�أ�
��3��ijԪ�ص����������Ų�ʽΪnsnnpn+1����n=2�����������Ų�ʽΪ2s22p3��ΪNԪ�أ���Ԫ�آ��γɵķ���XΪNH3��
��4��Ԫ�آڵ���������ΪBe��OH��2����NaOH��Һ��Ӧ��Ӧ����Na2BeO2��ˮ��
��5��Ԫ�آ��+3��������ΪAs2O3����ϡ������Һ�пɱ�Zn���ʻ�ԭ��������̬�⻯��AsH3�����ﻹ��������ZnSO4��ˮ����ƽ��д��Ӧ����ʽ��
��� �⣻��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪBe����ΪO����ΪMg����ΪAl����ΪS����ΪCl����ΪFe����ΪZn����ΪAs��
��1��d��Ԫ�ذ���3-10��Ԫ�أ�����������ϵԪ�ء��ϵԪ�س��⣩�����Ԫ�ش���d������ΪO�����ڵ������ڢ�A�壬�۵����Ų�ʽΪ2s22p4���۵����Ų�ͼΪ ����ΪZn��ԭ�Ӻ��������Ϊ30����������Ų�ʽΪ��1s22s22p63s23p63d104s2��
����ΪZn��ԭ�Ӻ��������Ϊ30����������Ų�ʽΪ��1s22s22p63s23p63d104s2��
�ʴ�Ϊ���� ��1s22s22p63s23p63d104s2��
��1s22s22p63s23p63d104s2��
��2��ͬ������ԭ����������Ԫ�ص�һ�����ܳ��������ƣ���MgԪ��ԭ��2sΪȫ���ȶ�״̬�������ϵͣ���һ�����ܸ���ͬ��������Ԫ�أ��ʵ�һ�����ܣ�Cl��S��Mg��Al���ʴ�Ϊ��Cl��S��Mg��Al��
��3��ijԪ�ص����������Ų�ʽΪnsnnpn+1����n=2�����������Ų�ʽΪ2s22p3��ΪNԪ�أ���Ԫ�آ��γɵķ���XΪNH3����ռ乹��Ϊ�����Σ��ʴ�Ϊ�������Σ�
��4��Ԫ�آڵ���������ΪBe��OH��2����NaOH��Һ��Ӧ��Ӧ����Na2BeO2��ˮ���÷�Ӧ����ʽΪ��Be��OH��2+2NaOH�TNa2BeO2+2H2O���ʴ�Ϊ��Be��OH��2+2NaOH�TNa2BeO2+2H2O��
��5��Ԫ�آ��+3��������ΪAs2O3����ϡ������Һ�пɱ�Zn���ʻ�ԭ��������̬�⻯��AsH3�����ﻹ��������ZnSO4��ˮ����Ӧ����ʽΪ��As2O3+6Zn+6H2SO4=2AsH3��+6ZnSO4+3H2O��
�ʴ�Ϊ��As2O3+6Zn+6H2SO4=2AsH3��+6ZnSO4+3H2O��
���� ���⿼��Ԫ�����ڱ���Ԫ�������ɡ���������Ų��������ܡ��ռ乹�͡�����ʽ��д�ȣ���Ҫѧ���߱���ʵ�Ļ������������֪ʶ�������Ѷ��еȣ�ע��ͬ�����е�һ�������쳣�����

 �㽭��У��ʦ���ϵ�д�
�㽭��У��ʦ���ϵ�д�| A�� | ����������������µĺڰ������в������ȶ����� | |
| B�� | ����ϩ����������ֱ�ͨ��������Ȼ�̼��Һ��ǰ����ɫ�����߲���ɫ | |
| C�� | ����ʹ��ˮ��ɫ����Ϊ���߷�Ӧ�������屽 | |
| D�� | Ҫ��ȥ�����е���ϩ�Ƶô��������飬�ɽ������ͨ�����Ը��������Һ�� |
| A�� | ��ʹ����KMn04��Һ��ɫ | B�� | �ܷ���������Ӧ | ||
| C�� | ����NaOH��Һ��Ӧ | D�� | �ܷ���ˮ�ⷴӦ |
�ټױ���������H2���ϣ�
�ڼ��������ҵ������ӷ���������ԭ��Ӧ
�ۼ��������Ӧ��ˮ�������Ա��ҵ��������Ӧ��ˮ��������ǿ��
����ij������Ӧʱ��ԭ�ӵõ�����Ŀ���ҵĶࣻ
�ݼ��⻯����ҵ��⻯���ȶ���
| A�� | �٢� | B�� | �ۢ� | C�� | �٢ڢ� | D�� | �٢ڢܢ� |
| A�� | ClO2-�Ŀռ乹��Ϊƽ�������� | |
| B�� | SiF4��SO32-������ԭ�Ӿ�Ϊsp3�ӻ� | |
| C�� | �����е�Ԫ���У����ĵ�һ��������� | |
| D�� | C2H5OH�����й�����8�����Լ���1���м� |
| A�� | ���ɵ�ص����������ǻ����Բ�ͬ�Ľ��� | |
| B�� | п�̸ɵ�ع���ʱ������ڲ��ĵ���������̼������пͰ | |
| C�� | �״�ȼ�ϵ�ع���ʱ���״��ڸ�������������Ӧ | |
| D�� | ��пŦ�۵�ع���ʱ��������п������������Һ���������� |
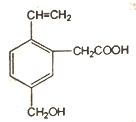
| A�� | ��ϩ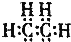 | B�� | �һ�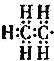 | C�� | ���Ȼ�̼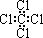 | D�� | 2-����ϩ |
| A�� | ��طŵ�ʱ��H2���븺����Ӧ������������Ӧ | |
| B�� | ��طŵ�ʱ����Ԫ�ر����� | |
| C�� | ��طŵ�ʱ�����Ӵ�������һ����NiO��OH�� ��һ�� | |
| D�� | ��س��ʱ������ת��Ϊ��ѧ�� |