��Ŀ����
��14�֣�����KMnO4��Һ������ᣨH2C2O4����Һ��Ӧ��ij̽��С�����÷�Ӧ��������Һ��ɫ��ʧ�����ķ������о�Ӱ�췴Ӧ���ʵ����ء�
��.ʵ��ǰ������Ũ��Ϊ0.1000mol?L-1����KMnO4����Һ�ζ�δ֪Ũ�ȵIJ��ᡣ
��1��д���ζ������з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2���ζ������в����ζ��ܵ�ͼʾ��ȷ���� ��

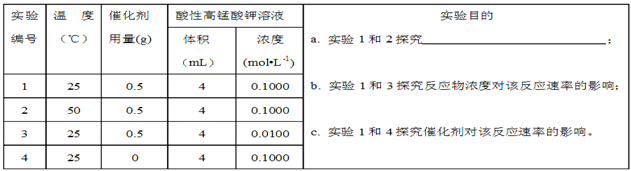
��3�����ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ����ʹ��õIJ�����ҺŨ�� ���ƫ�ߡ�����ƫ�͡������䡱����
��.ͨ���ζ�ʵ��õ�������Һ��Ũ��Ϊ0.2000mol��L-1 ���øò�����Һ���±����к���ʵ�飨ÿ��ʵ�������Һ��������Ϊ8mL����

��4��д������a ��Ӧ��ʵ��Ŀ�� ����50��Cʱ������Ũ��c(H2C2O4)�淴Ӧʱ��t�ı仯���� ����ͼ��ʾ�����������������䣬����ͼ�л���25��Cʱc(H2C2O4)��t�ı仯����ʾ��ͼ��

��5����С��ͬѧ��ʵ��1��3�ֱ����������ʵ�飬�������ʵ�����ݣ��ӻ�����ȿ�ʼ��ʱ����
�����������ݺ�ó���������������ͬʱ�����Ը��������Һ��Ũ��ԽС����ɫʱ���Խ�̣�����Ӧ���ʾ�Խ�족�Ľ��ۡ���ͬѧ��Ϊ��С�顰̽����Ӧ��Ũ�ȶ�����Ӱ�족��ʵ�鷽������д������⣬�Ӷ��õ��˴����ʵ����ۣ��������ͬѧ�Ľ���ʵ�鷽�� ______________________��
��6����ʵ����ʹ�õĴ���Ӧѡ��MnSO4����MnCl2��ԭ��������ӷ���ʽ��ʾΪ ��
��.ʵ��ǰ������Ũ��Ϊ0.1000mol?L-1����KMnO4����Һ�ζ�δ֪Ũ�ȵIJ��ᡣ
��1��д���ζ������з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2���ζ������в����ζ��ܵ�ͼʾ��ȷ���� ��


��3�����ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ����ʹ��õIJ�����ҺŨ�� ���ƫ�ߡ�����ƫ�͡������䡱����
��.ͨ���ζ�ʵ��õ�������Һ��Ũ��Ϊ0.2000mol��L-1 ���øò�����Һ���±����к���ʵ�飨ÿ��ʵ�������Һ��������Ϊ8mL����

��4��д������a ��Ӧ��ʵ��Ŀ�� ����50��Cʱ������Ũ��c(H2C2O4)�淴Ӧʱ��t�ı仯���� ����ͼ��ʾ�����������������䣬����ͼ�л���25��Cʱc(H2C2O4)��t�ı仯����ʾ��ͼ��

��5����С��ͬѧ��ʵ��1��3�ֱ����������ʵ�飬�������ʵ�����ݣ��ӻ�����ȿ�ʼ��ʱ����
�����������ݺ�ó���������������ͬʱ�����Ը��������Һ��Ũ��ԽС����ɫʱ���Խ�̣�����Ӧ���ʾ�Խ�족�Ľ��ۡ���ͬѧ��Ϊ��С�顰̽����Ӧ��Ũ�ȶ�����Ӱ�족��ʵ�鷽������д������⣬�Ӷ��õ��˴����ʵ����ۣ��������ͬѧ�Ľ���ʵ�鷽�� ______________________��
��6����ʵ����ʹ�õĴ���Ӧѡ��MnSO4����MnCl2��ԭ��������ӷ���ʽ��ʾΪ ��
��1�� 2MnO4��+5H2C2O4+6H+= 2Mn2+ + 10CO2 ��+ 8H2O ����2�� A����3�� ƫ�� ��
��4��̽���¶Ȳ�ͬ�Է�Ӧ���ʵ�Ӱ��
25��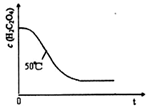
��5������������ͬʱ�����õ����������ĸ��������������ͬŨ�ȵ�����������Һ��Ӧ��������Һ��ɫʱ�䡣
��6��2MnO4��+ 10Cl�� + 16H+ = 5Cl2��+ 2Mn2+ + 8H2O
��4��̽���¶Ȳ�ͬ�Է�Ӧ���ʵ�Ӱ��
25��

��5������������ͬʱ�����õ����������ĸ��������������ͬŨ�ȵ�����������Һ��Ӧ��������Һ��ɫʱ�䡣
��6��2MnO4��+ 10Cl�� + 16H+ = 5Cl2��+ 2Mn2+ + 8H2O
�����������1�� ����KMnO4��ǿ�����ԣ��������л�ԭ�ԣ����߷���������ԭ��Ӧ�����ӷ���ʽΪ�� 2MnO4��+5H2C2O4+6H+= 2Mn2+ + 10CO2 ��+ 8H2O ����2���ζ������в�����ʽ�ζ��ܵķ�����ͼʾ��A����3�����ζ�ǰ�ζ��ܼ��촦�����ݣ��ζ�����ʧ�������ĵ�����KMnO4����Һ����ƫ���Դ�Ϊ������IJ�����ҺŨ�� ��ƫ�ߡ���4������a���Ӧ��������������ͬ��ֻ���¶Ȳ�ͬ����˱���a ��Ӧ��ʵ��Ŀ����̽���¶Ȳ�ͬ�Է�Ӧ���ʵ�Ӱ�졣��5����ͬѧ�Ľ���ʵ�鷽��������������ͬʱ�����õ����������ĸ��������������ͬŨ�ȵ�����������Һ��Ӧ��������Һ��ɫʱ�䡣 ��Һ��ɫʱ��Խ�̣���Ӧ����Խ�졣��6����ʵ����ʹ�õĴ���Ӧѡ��MnSO4����MnCl2��ԭ����Cl-�л�ԭ�ԣ�Ҳ��������KMnO4����Һ��Ӧ����Ӱ��ʵ�����IJⶨ���������ӷ���ʽ��ʾΪ2MnO4�� + 10Cl�� + 16H+ = 5Cl2��+ 2Mn2+ + 8H2O ��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
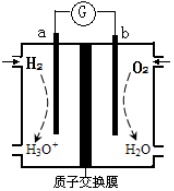
 4C(?)+2D(?)����Ӧһ��ʱ���ﵽƽ�⣬�������1��6 mol C���ҷ�Ӧ��ǰ��ѹǿ֮��Ϊ5��4(��ͬ���¶��²���)��������˵����ȷ����( )
4C(?)+2D(?)����Ӧһ��ʱ���ﵽƽ�⣬�������1��6 mol C���ҷ�Ӧ��ǰ��ѹǿ֮��Ϊ5��4(��ͬ���¶��²���)��������˵����ȷ����( )
 2NO���ܱ������н���,���д�ʩ������Ӧ���ʵ��� ( )��
2NO���ܱ������н���,���д�ʩ������Ӧ���ʵ��� ( )��