��Ŀ����
17��������������ɫ�ϳ�·��֮һΪͼ1��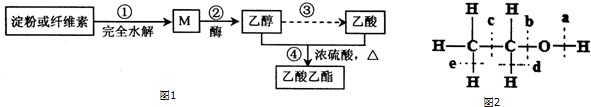
��1��M�Ľṹ��ʽΪHOCH2��CHOH��4CHO������˵������ȷ����C��
A�����ۺ���ά�ض�������Ȼ�߷��ӻ�����
B��M��������������ͭ����Һ�ڼ�������������ש��ɫ����
C�����ۺ���ά�صĻ�ѧʽ��Ϊ��C6H10O5��n������Ϊͬ���칹��
D���ñ���̼������Һ���Լ����Ҵ����������������
��2���Ҵ����ӽṹ�л�ѧ����ͼ2��
���Ҵ��ͽ����Ʒ�Ӧʱ�����ѵĻ�ѧ����a������ĸ������Ӧ�Ļ�ѧ����ʽ��2CH3CH2OH+2Na��2CH3CH2ONa+H2����
���Ҵ���ͭ������ʱ��������Ӧ�����ѵĻ�ѧ����ad������ĸ������Ӧ�Ļ�ѧ����ʽ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
��3���������е�ԭ����-COOH���������Ȼ���
����ʾ��ԭ�ӷ���ȷ��ijЩ��ѧ��Ӧ�Ļ�����д����CH3CH2COOH�� CH3CH218OH��Ӧ��ȡ���Ļ�ѧ����ʽCH3CH218OH+CH3 CH2COOH
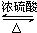 CH3 CH2CO18OCH2CH3+H2O��
CH3 CH2CO18OCH2CH3+H2O�����������������ķ�Ӧ�ǿ��淴Ӧ��������˵���÷�Ӧ�Ѵﵽ��ѧƽ��״̬����BD������ţ�
A����λʱ�������1mol����������ͬʱ����1molˮ
B����λʱ�������1mol����������ͬʱ����1mol����
C����λʱ�������1mol�Ҵ���ͬʱ����1mol����
D������Ӧ���������淴Ӧ��������ȣ�
���� ��1�����ۻ���ά����ȫˮ��õ�MΪ�����ǣ��������ھƻ�ø�����µõ�CH3CH2OH��CH3CH2OH����������Ӧ�õ�CH3COOH��CH3COOH��CH3CH2OH����������Ӧ�õ�CH3COOCH2CH3��
��2�����Ҵ����Ʒ�Ӧ�����Ҵ��������������Ҵ�����������������ȩ���Ա��л���Ľṹ�ж϶��ѵĻ�ѧ����
��3���������е�ԭ����-COOH���������Ȼ���
�������봼����������Ӧ�������ṩ�ǻ������ṩ�ǻ��⣬��������ˮ���������Ž����������
�ۿ��淴Ӧ�õ�ƽ��ʱ��ͬ�����ʵ���������������������ȣ��ݴ��жϣ�
��� �⣺��1�����ۻ���ά����ȫˮ��õ�MΪ�����ǣ��ṹ��ʽΪHOCH2��CHOH��4CHO���������ھƻ�ø�����µõ�CH3CH2OH��CH3CH2OH����������Ӧ�õ�CH3COOH��CH3COOH��CH3CH2OH����������Ӧ�õ�CH3COOCH2CH3��
A�����ۺ���ά�ػ�ѧʽ���ɱ�ʾΪ��C6H10O5��n����������Ȼ�߷��ӻ������A��ȷ��
B��MΪ�����ǣ�����ȩ������������������ͭ����Һ�ڼ�������������ש��ɫ��������B��ȷ��
C�����ۺ���ά�صĻ�ѧʽ��Ϊ��C6H10O5��n���ۺ϶Ȳ�ͬ�����߲���ͬ���칹�壬��C����
D������������̼������Һ�зֲ㣬�Ҵ������������̼������Һ����������̼���Ʒ�Ӧ���ɶ�����̼���������ͬ�����Խ��м��𣬹�D��ȷ��
�ʴ�Ϊ��HOCH2��CHOH��4CHO��C��
��2�����Ҵ����Ʒ�Ӧ�����Ҵ�������������Ӧ����ʽΪ��2CH3CH2OH+2Na��2CH3CH2ONa+H2�����Ҵ���O-H����ѣ�
�ʴ�Ϊ��a�� 2CH3CH2OH+2Na��2CH3CH2ONa+H2����
���Ҵ�����������������ȩ����Ӧ����ʽΪ��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O���Ҵ���O-H�����ѡ��ǻ����ӵ�̼ԭ���е�C-H�����ѣ�
�ʴ�Ϊ��ad��2CH3CH2OH+O2$��_{��}^{Cu}$2CH3CHO+2H2O��
��3���������е�ԭ����-COOH���������Ȼ����ʴ�Ϊ���Ȼ���
�������봼����������Ӧ�������ṩ�ǻ������ṩ�ǻ��⣬��������ˮ���������Ž������������Ӧ����ʽΪ��CH3CH218OH+CH3 CH2COOH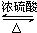 CH3 CH2CO18OCH2CH3+H2O��
CH3 CH2CO18OCH2CH3+H2O��
�ʴ�Ϊ��CH3CH218OH+CH3 CH2COOH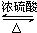 CH3 CH2CO18OCH2CH3+H2O��
CH3 CH2CO18OCH2CH3+H2O��
��A����λʱ�������1mol����������ͬʱ����1molˮ������ʾ����Ӧ���ʣ���Ӧʼ�հ��ñ�����ϵ���У�����˵������ƽ�⣬��A����
B����λʱ�������1mol����������ͬʱ����1mol���ᣬ������1mol����ͬʱ����1mol����������˵����Ӧ����ƽ�⣬��B��ȷ��
C����λʱ�������1mol�Ҵ���ͬʱ����1mol���ᣬ����ʾ����Ӧ���ʣ���Ӧʼ�հ��ñ�����ϵ���У�����˵������ƽ�⣬��C����
D������Ӧ���������淴Ӧ��������ȣ���Ӧ�õ�ƽ�⣬��D��ȷ��
��ѡ��BD��
���� ��������ƴ������Ŀ���漰�л�����ƶ���ϳɡ��л���Ľṹ�����ʡ�ƽ��״̬���жϣ����ضԻ���֪ʶ�Ĺ��̣�

 �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д� ������״Ԫ��ҵϵ�д�
������״Ԫ��ҵϵ�д�| A�� | ����HI��Һ���뵽Fe��NO3��3��Һ�У�2Fe3++2I-�T2Fe2++I2 | |
| B�� | �������ʵ�����MgCl2��Ba��OH��2��HNO3������Һ��ϣ�Mg2++2OH-�TMg��OH��2�� | |
| C�� | CaCO3���ڴ��CaCO3+2CH3COOH�TCa2++2CH3COO-+CO2��+2H2O | |
| D�� | ���ҽ���Һ�м������ᡢ˫��ˮ��2I-+H2O2�T2OH-+I2 |
| A�� | ������0.10 mol/L CH3COOH��Һ���ٲ���Һ��pH����pH����1�����֤������Ϊ������� | |
| B�� | ������0.01 mol/L��0.10 mol/L��CH3COOH���ٷֱ���pH�Ʋ����ǵ�pH�������ߵ�pH���С��1����λ�����֤��������������� | |
| C�� | ȡ���������pH��CH3COOH����Һ������ֱ�������п��Ӧ����÷�Ӧ�����д������H2���ʽ��������ղ���H2�϶࣬���֤������Ϊ������� | |
| D�� | ����һ������CH3COONa��Һ������pH����������pH����7�����֤��������������� |
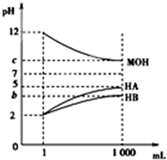 �����£���pH=2����������ҺHA��HB��pH=12�ļ���ҺMOH��1mL���ֱ��ˮϡ�͵�1 000mL����pH�ı仯����Һ����Ĺ�ϵ��ͼ������˵��������ǣ�������
�����£���pH=2����������ҺHA��HB��pH=12�ļ���ҺMOH��1mL���ֱ��ˮϡ�͵�1 000mL����pH�ı仯����Һ����Ĺ�ϵ��ͼ������˵��������ǣ�������| A�� | HAΪǿ�ᣬHBΪ���� | |
| B�� | ��b+c=14����MOH���� | |
| C�� | ��c=9����ϡ�ͺ��������Һ�У���ˮ����������ӵ�Ũ�ȵĴ�С˳��ΪHA��MOH��HB | |
| D�� | ��c=9����ϡ�ͺ��HA��Һ��MOH��Һȡ�������ϣ���������Һ��c��A-��=c��M+�� |
| A�� | ��������к����Ȼ�������NaHCO3��Һ��Ӧ���� CO2 | |
| B�� | ���еĴ���ͭ���������ȵ������£�������O2 �������� | |
| C�� | ����������ڹ����µķ�Ӧ�뱽��������Ӧ�ķ�Ӧ������ͬ | |
| D�� | ������ʹ������Ȼ�̼��Һ��ɫ��˵����������û��̼̼˫�� |
 ��
�� ����пƬ�ʹ�ͭƬ����ͼ��ʽ����100mL��ͬŨ�ȵ�ϡ������һ��ʱ�䣬�ش��������⣺
����пƬ�ʹ�ͭƬ����ͼ��ʽ����100mL��ͬŨ�ȵ�ϡ������һ��ʱ�䣬�ش��������⣺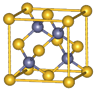 ��λ�ڵ������ڢ�B�壬��Ҫ���ϼ�+2��+3��+6������Ӳ�ȴ���ʴ������Ҫ�ĺϽ���ϣ�
��λ�ڵ������ڢ�B�壬��Ҫ���ϼ�+2��+3��+6������Ӳ�ȴ���ʴ������Ҫ�ĺϽ���ϣ�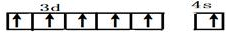 ��CrO2Cl2������Ϊ���ɫҺ�壬����CCl4��CS2�Ȼ��ܣ��ݴ˿��ж�CrO2Cl2�ǷǼ��ԣ�����ԡ��Ǽ��ԡ������ӣ�
��CrO2Cl2������Ϊ���ɫҺ�壬����CCl4��CS2�Ȼ��ܣ��ݴ˿��ж�CrO2Cl2�ǷǼ��ԣ�����ԡ��Ǽ��ԡ������ӣ�