��Ŀ����
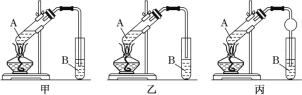
����Ŀ����1��ijͬѧ������ȡ����Һ�ķ����ӵ�ˮ����ȡ�⣬��Ҫ������������ͼ��
�ף�������ȡ������ �ң����÷ֲ� ��������
�ٸ�ͬѧ�����õ���ȡ��������______������A������B������C������
A���ƾ� B���� C�����Ȼ�̼
�ڼס��ҡ���3��ʵ������У�����ȷ����______������������������������������
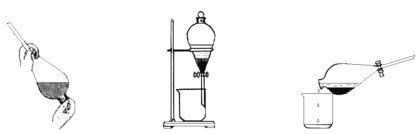
��2��ʵ������ȡ���������ռ������漰���¼�����Ҫ�Σ�

��������2���Լ���
A������ʳ��ˮ B��NaOH��Һ
���������Լ���ѡ����գ�����A������B������
��X��ҺӦѡ��________________��
��β�����������У��������������________________���ա�
���𰸡�C �� A B
��������
(1)�����ȡ����Һ����Ҫ��������⣻
(2)��ͼ��֪������������Ũ���ᷴӦ��������������HCl��ͨ��X(����ʳ��ˮ)��ȥHCl��Ȼ��ͨ��Ũ����������������������ſ����ռ���β������NaOH��Һ���գ��Դ������
(1)�ٴӵ�ˮ����ȡ�⣬��ȡ��������ˮ���ܣ����ƾ���ˮ���ܣ���������ȡ��������ѡ��CCl4����ȡ�������б����ܶȱ�ˮС����ȡ���л������ϲ㣬��ֻ��ѡ��CCl4����ȡ�����ʴ�ΪC��
�������÷ֲ�ٳֺ�����������ʱ�ȷ����²�Һ����ϲ�Һ�壬���ͼ���������ʴ�Ϊ����
(2)��ͼ��֪������������Ũ���ᷴӦ��������������HCl��ͨ��X(����ʳ��ˮ)��ȥHCl��Ȼ��ͨ��Ũ����������������������ſ����ռ���β������NaOH��Һ���գ�
��������������֪��XΪ�����Լ���Ϊ����ʳ��ˮ���������������ܽ⣬�ʴ�ΪA��
��������NaOH��Ӧ���������ʣ���������������NaOH���գ��ʴ�ΪB��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�