��Ŀ����
�о���Աͨ���Ա�������PM2��5�Ļ�ѧ����о����֣�����β����ȼú��Ⱦ�ֱ� ռ4����l8��
I����1�����ھ�������β���ķ�ӦΪ��2NO(g)+2CO(g) 2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ�
2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ�
��2���������û���̿��ԭ���������������ӦΪ��C(s)+2NO(g) N2(g)+CO2(g)
N2(g)+CO2(g)  H=akJ��mol-1����ij�ܱ���������һ�����Ļ���̿��NO������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
H=akJ��mol-1����ij�ܱ���������һ�����Ļ���̿��NO������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
��T1��ʱ���÷�Ӧ��ƽ�ⳣ��K= (������λС��)��
��ǰ10min����v(NO)��ʾ�Ļ�ѧ��Ӧ����Ϊ ��30min�ı�ijһ��������Ӧ���´ﵽƽ�⣬��ı������������ ��
����30min�������¶���T2�棬�ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ3��1��l����÷�Ӧ��a 0(�>������=����<��)��
��CO���������滷����Ӱ��ܴ�CO�����������ڵ��������ȵ����⡣
��1����ҵ�ϳ���SO2��ȥCO��������ΪS��CO2����֪��ط�Ӧ���̵������仯��ͼ��ʾ

����SO2��ȥCO���Ȼ�ѧ����ʽΪ ��
��2������ʱ��Ҳ������CO��ԭMgSO4���Ʊ��ߴ�MgO��
��750��ʱ����������к������ʵ���SO2��SO3���˷�Ӧ�Ļ�ѧ����ʽ�� ��
����Mg���Ƴɡ�þһ�������Ρ���أ���װ��ʾ��ͼ��ͼ����þ�缫�����ĵ缫��ӦʽΪ ���õ���ܷ�Ӧ�����ӷ���ʽΪ ��

I����1�����ھ�������β���ķ�ӦΪ��2NO(g)+2CO(g)
 2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ�
2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ� | A��װ��β������װ�õ������ų��������в��ٺ���NO��CO |
| B�����β������Ч�ʵ����;����ʹ�ø�Ч���� |
| C������ѹǿ������ƽ�����ƣ���ʵ�ʲ����п�ͨ����ѹ�ķ�ʽ����侻��Ч�� |
| D�����β������Ч�ʵij��÷����������¶� |
 N2(g)+CO2(g)
N2(g)+CO2(g)  H=akJ��mol-1����ij�ܱ���������һ�����Ļ���̿��NO������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
H=akJ��mol-1����ij�ܱ���������һ�����Ļ���̿��NO������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�| Ũ��/mol��L-1 ʱ��/min | NO | N2 | CO2 |
| 0 | 0.100 | 0 | 0 |
| 10 | 0.058 | 0.021 | 0.021 |
| 20 | 0.050 | 0.025 | 0.025 |
| 30 | 0.050 | 0.025 | 0.025 |
| 40 | 0.036 | 0.032 | 0.010 |
| 50 | 0.036 | 0.032 | 0.010 |
��T1��ʱ���÷�Ӧ��ƽ�ⳣ��K= (������λС��)��
��ǰ10min����v(NO)��ʾ�Ļ�ѧ��Ӧ����Ϊ ��30min�ı�ijһ��������Ӧ���´ﵽƽ�⣬��ı������������ ��
����30min�������¶���T2�棬�ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ3��1��l����÷�Ӧ��a 0(�>������=����<��)��
��CO���������滷����Ӱ��ܴ�CO�����������ڵ��������ȵ����⡣
��1����ҵ�ϳ���SO2��ȥCO��������ΪS��CO2����֪��ط�Ӧ���̵������仯��ͼ��ʾ

����SO2��ȥCO���Ȼ�ѧ����ʽΪ ��
��2������ʱ��Ҳ������CO��ԭMgSO4���Ʊ��ߴ�MgO��
��750��ʱ����������к������ʵ���SO2��SO3���˷�Ӧ�Ļ�ѧ����ʽ�� ��
����Mg���Ƴɡ�þһ�������Ρ���أ���װ��ʾ��ͼ��ͼ����þ�缫�����ĵ缫��ӦʽΪ ���õ���ܷ�Ӧ�����ӷ���ʽΪ ��

I��1��B ��2���� 0.25 �� 0.0042mol???L-1?min-1������CO2Ũ�� �� <
II��1��SO2(g)+2CO(g)=S(s)+2CO2(g) ?H=(2b��a)kJ?mol?1
��2����2MgSO4+CO 2MgO+SO2+CO2+SO3
2MgO+SO2+CO2+SO3
�� Mg��2e?+2OH?=Mg(OH)2��Mg��2e?=Mg2+ Mg+ClO?+H2O=Cl?+Mg(OH)2
II��1��SO2(g)+2CO(g)=S(s)+2CO2(g) ?H=(2b��a)kJ?mol?1
��2����2MgSO4+CO
 2MgO+SO2+CO2+SO3
2MgO+SO2+CO2+SO3�� Mg��2e?+2OH?=Mg(OH)2��Mg��2e?=Mg2+ Mg+ClO?+H2O=Cl?+Mg(OH)2
���������I��1���÷�Ӧ��ƽ�ⳣ���Ѿ��ܴ��ˣ��������β������Ч�ʹؼ��Ǽӿ췴Ӧ���ʡ�A����Ϊ�÷�ӦΪ���淴Ӧ������װ��β������װ�õ������ų�����������Ȼ����NO��CO������B��ʹ�ø�Ч�������Լӿ췴Ӧ���ʣ��������β������Ч�ʵ����;����ʹ�ø�Ч��������ȷ��C������ѹǿ������β�����ŷź�������������D�������¶ȣ�����β�����ŷź�������������
��2���ٷ�Ӧ���е�20minʱ�ﵽƽ�⣬��������ʽ���м���:
C(s)+2NO(g)
 N2(g)+CO2(g)
N2(g)+CO2(g)��ʼŨ�ȣ�mol?L?1�� 0.100 0 0
ת��Ũ�ȣ�mol?L?1�� 0.050 0.025 0.025
ƽ��Ũ�ȣ�mol?L?1�� 0.050 0.025 0.025
�÷�Ӧ��ƽ�ⳣ��K= 0.025��0.025��0.0502=0.25��
�� v(NO)=(0.1000mol?L?1��0.058mol?L?1)��10min =0.0042mol???L-1?min-1��30min�ı�ijһ��������Ӧ���´ﵽƽ���Ӧ��NO��Ũ�ȼ�С��������N2Ũ������CO2��Ũ�ȼ�С�����Ըı�����������ǣ�����CO2Ũ�ȡ�
�� T1�� 30minʱ��NO��N2��CO2��Ũ��֮��Ϊ2:1:1�������¶���T2�棬�ﵽƽ��ʱ��������NO��N2��CO2��Ũ��֮��Ϊ3��1��l��˵�������¶ȣ�ƽ�����淴Ӧ�����ƶ����÷�ӦΪ���ȷ�Ӧ��a < 0
II��1�����������仯ͼ�ɵ��Ȼ�ѧ����ʽ��S(s)+O2(g)=SO2(g) ?H1="a" kJ?mol?1 CO(g)+1/2O2(g)=CO2(g) ?H2="b" kj/mol��д����SO2��ȥCO�Ļ�ѧ����ʽ��ע��״̬�����ݸ�˹�������ʱ䣬?H=��?H1+ 2?H2 = =(2b��a)kJ?mol?1���ɵ��Ȼ�ѧ����ʽ��
��2����CO��ԭMgSO4���Ʊ��ߴ�MgO�����ɵ����ʵ�����SO2��SO3������Ԫ���غ�ͬʱ����CO2����ƽ�ɵû�ѧ����ʽ��2MgSO4+CO
 2MgO+SO2+CO2+SO3��
2MgO+SO2+CO2+SO3���� MgΪ���ý�����Ϊ����ʧȥ���ӣ��缫����ʽΪ��Mg��2e?+2OH?=Mg(OH)2��Mg��2e?=Mg2+���ܷ�ӦΪClO?�ڼ��������°�Mg����ΪMg(OH)2�����ӷ���ʽΪ��Mg+ClO?+H2O=Cl?+Mg(OH)2

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 CH3OCH3(g)+H2O(g) ��H="-25" kJ/mol��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£���1 L���ܱ������м���CH3OH,��Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�������
CH3OCH3(g)+H2O(g) ��H="-25" kJ/mol��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£���1 L���ܱ������м���CH3OH,��Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�������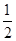 O2(g)=H2O(l) ��H����286 kJ/mol���ж�H2O������O��H���ļ���Ϊ( )
O2(g)=H2O(l) ��H����286 kJ/mol���ж�H2O������O��H���ļ���Ϊ( ) SO2(g)����H=a kJ��mol-1(a=-297.2)����������˵��,���в���ȷ����(����)
SO2(g)����H=a kJ��mol-1(a=-297.2)����������˵��,���в���ȷ����(����)