��Ŀ����
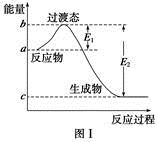
��һ�������£��״����ڻ�ѧƽ�⣺2CH3OH(g) CH3OCH3(g)+H2O(g) ��H="-25" kJ/mol��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£���1 L���ܱ������м���CH3OH,��Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�������
CH3OCH3(g)+H2O(g) ��H="-25" kJ/mol��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£���1 L���ܱ������м���CH3OH,��Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�������
����˵����ȷ����
A��ƽ��ʱCH3OH�� ���ʵ���Ũ��Ϊ0.8mol/L
B�������¶ȣ�����Ӧ���ʼ�С���淴Ӧ��������ƽ�����淴Ӧ�����ƶ�
C���Ӵ�ʱ�̵�ƽ��ʱ����Ӧ�ų�����9 kJ
D��ƽ��ʱ���ټ�������ʼ������CH3OH,����ƽ���CH3OHת���ʼ�С
 CH3OCH3(g)+H2O(g) ��H="-25" kJ/mol��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£���1 L���ܱ������м���CH3OH,��Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�������
CH3OCH3(g)+H2O(g) ��H="-25" kJ/mol��ij�¶��µ�ƽ�ⳣ��Ϊ400�����¶��£���1 L���ܱ������м���CH3OH,��Ӧ��ijʱ�̲�ø���ֵ����ʵ���Ũ�������| ���� | CH3OH | CH3OCH3 | H2O |
| C/(mo/L) | 0.8 | 1.24 | 1.24 |
A��ƽ��ʱCH3OH�� ���ʵ���Ũ��Ϊ0.8mol/L
B�������¶ȣ�����Ӧ���ʼ�С���淴Ӧ��������ƽ�����淴Ӧ�����ƶ�
C���Ӵ�ʱ�̵�ƽ��ʱ����Ӧ�ų�����9 kJ
D��ƽ��ʱ���ټ�������ʼ������CH3OH,����ƽ���CH3OHת���ʼ�С
C
���������A����Ӧ���е�c(CH3OH)=0.8mol?L?1ʱ��Ũ����Q=1.24��1.24��0.82="2.4" < K����Ӧ��δ�ﵽƽ�⣬����ƽ��Ũ��Ӧ����0.8mol?L?1,����B�������¶ȣ�����Ӧ���ʺ��淴Ӧ���ʶ�������C����ﵽƽ���CH3OH����ת����Ũ��Ϊ2xmol/L����������ʽ���м��㣺
2CH3OH(g)
 CH3OCH3(g) + H2O(g)
CH3OCH3(g) + H2O(g)ijʱ�� 0.8 mol��L-1 1.24 mol��L-1 1.24 mol��L-1
�仯 2x mol��L-1 x mol��L-1 x mol��L-1
ƽ�� (0.8-2x) mol��L-1 1.24+x mol��L-1 1.24+x mol��L-1
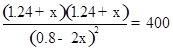 �����x="0.36" mol/L��ƽ��ʱ����Ӧ��״����������ĵ�0.72 mol���ų�����Ϊ12.5 kJ/mol��0.72 mol="9" kJ,��ȷ��D��ƽ��ʱ���ټ�������ʼ������CH3OH,����ƽ��ʱ��ԭƽ���Ч��CH3OHת���ʲ��䣬����
�����x="0.36" mol/L��ƽ��ʱ����Ӧ��״����������ĵ�0.72 mol���ų�����Ϊ12.5 kJ/mol��0.72 mol="9" kJ,��ȷ��D��ƽ��ʱ���ټ�������ʼ������CH3OH,����ƽ��ʱ��ԭƽ���Ч��CH3OHת���ʲ��䣬����
��ϰ��ϵ�д�
 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д�
�����Ŀ
 2NH3(g)����H="-92.4" kJ��mol-1,��N��N���ļ�����(����)
2NH3(g)����H="-92.4" kJ��mol-1,��N��N���ļ�����(����) 2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ�
2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ�  N2(g)+CO2(g)
N2(g)+CO2(g)  H=akJ��mol-1����ij�ܱ���������һ�����Ļ���̿��NO������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
H=akJ��mol-1����ij�ܱ���������һ�����Ļ���̿��NO������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�