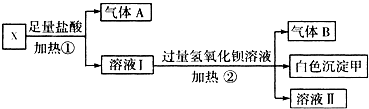
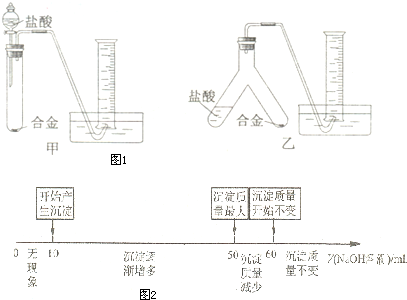
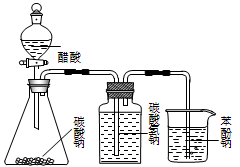
��Ŀ����
����һ�������δ�����п��ܺ���CaCO3��Na2CO3��Na2SO4��NaCl��CuSO4���ֽ�������ʵ�飺
������ˮ����ɫ��Һ��
������Һ�м����Ȼ�����Һ���ɰ�ɫ�������ټ�����ʱ������ʧ��
��������ʵ�������ƶϣ�
��1��һ�������ڵ������ǣ�______��
��2��һ�����ڵ������ǣ�______��
��3�����ܴ��ڵ������ǣ�______��
��4�����ڿ��ܴ��ڵ����ʵļ��鷽���ǣ�д����Ҫ�IJ�����йص����ӷ���ʽ����______��
������ˮ����ɫ��Һ��
������Һ�м����Ȼ�����Һ���ɰ�ɫ�������ټ�����ʱ������ʧ��
��������ʵ�������ƶϣ�
��1��һ�������ڵ������ǣ�______��
��2��һ�����ڵ������ǣ�______��
��3�����ܴ��ڵ������ǣ�______��
��4�����ڿ��ܴ��ڵ����ʵļ��鷽���ǣ�д����Ҫ�IJ�����йص����ӷ���ʽ����______��
��1����ԭ��Һ��CaCO3������ˮ��CuSO4����ˮ��Ϊ��ɫҺ�壬����ԭ�����ĩ��һ���������������ʣ�
�ڵμӹ�����BaCl2��Һ�����ְ�ɫ��������ɫ����������̼�ᱵ�����ᱵ��̼�ᱵ�������ᣬ���ᱵ���ܣ�������ʱ������ʧ��˵����̼�ᱵ������һ������̼������ӣ�һ����������������ӣ���һ�������ڵ�������CaCO3��Na2SO4��CuSO4��һ����Na2CO3��
�ʴ�Ϊ��CaCO3��Na2SO4��CuSO4��
��2���������Ϸ���һ�����ڵ������ǣ�Na2CO3��
�ʴ�Ϊ��Na2CO3��
��3�����ܴ��ڵ�������NaCl��
�ʴ�Ϊ��NaCl��
��4���Ȼ��ƵĴ��������ͨ��������������ȷ���������ӵļ�������������ữ�������������飬�����ǣ�ȡ�����δ��������ˮ�ܽ����ϡ�����ữ���ټ���������Һ������а�ɫ�������ɣ���˵������NaCl�������ķ�ӦΪCO32-+2H+�TCO2��+H2O��Ag++Cl-�TAgCl����
�ʴ�Ϊ��ȡ�����δ��������ˮ�ܽ����ϡ�����ữ���ټ���������Һ������а�ɫ�������ɣ���˵������NaCl��CO32-+2H+�TCO2��+H2O��Ag++Cl-�TAgCl����
�ڵμӹ�����BaCl2��Һ�����ְ�ɫ��������ɫ����������̼�ᱵ�����ᱵ��̼�ᱵ�������ᣬ���ᱵ���ܣ�������ʱ������ʧ��˵����̼�ᱵ������һ������̼������ӣ�һ����������������ӣ���һ�������ڵ�������CaCO3��Na2SO4��CuSO4��һ����Na2CO3��
�ʴ�Ϊ��CaCO3��Na2SO4��CuSO4��
��2���������Ϸ���һ�����ڵ������ǣ�Na2CO3��
�ʴ�Ϊ��Na2CO3��
��3�����ܴ��ڵ�������NaCl��
�ʴ�Ϊ��NaCl��
��4���Ȼ��ƵĴ��������ͨ��������������ȷ���������ӵļ�������������ữ�������������飬�����ǣ�ȡ�����δ��������ˮ�ܽ����ϡ�����ữ���ټ���������Һ������а�ɫ�������ɣ���˵������NaCl�������ķ�ӦΪCO32-+2H+�TCO2��+H2O��Ag++Cl-�TAgCl����
�ʴ�Ϊ��ȡ�����δ��������ˮ�ܽ����ϡ�����ữ���ټ���������Һ������а�ɫ�������ɣ���˵������NaCl��CO32-+2H+�TCO2��+H2O��Ag++Cl-�TAgCl����

��ϰ��ϵ�д�
�����Ŀ