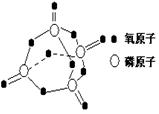
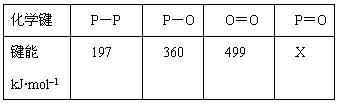
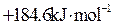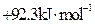��Ŀ����
��3�֣���֪����298K��100kPaʱ��
��C(s,ʯī)��O2(g) = CO2(g) ��H1 = ��400 kJ��mol��1��
��2H2(g)��O2(g) = 2H2O(l) ��H2 = ��570 kJ��mol��1��
��2C2H2(g)��5O2(g) = 4CO2(g)+ 2H2O(l) ��H3 = ��2600 kJ��mol��1��
д��298Kʱ��C(s,ʯī)��H2(g)����1 mol C2H2(g)��Ӧ���Ȼ�ѧ����ʽ ��
��
��C(s,ʯī)��O2(g) = CO2(g) ��H1 = ��400 kJ��mol��1��
��2H2(g)��O2(g) = 2H2O(l) ��H2 = ��570 kJ��mol��1��
��2C2H2(g)��5O2(g) = 4CO2(g)+ 2H2O(l) ��H3 = ��2600 kJ��mol��1��
д��298Kʱ��C(s,ʯī)��H2(g)����1 mol C2H2(g)��Ӧ���Ȼ�ѧ����ʽ
 ��
��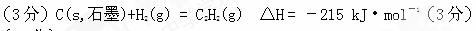
��

��ϰ��ϵ�д�
 ������ȫ�̼����ĩ���100��ϵ�д�
������ȫ�̼����ĩ���100��ϵ�д�
�����Ŀ
 2SO3( g ) ���� H =-QkJ��mol-1�����������·ֱ���������ͷ�Ӧ�ų������� ( Q)���±����У������������ݣ�����������ȷ���ǣ� ��
2SO3( g ) ���� H =-QkJ��mol-1�����������·ֱ���������ͷ�Ӧ�ų������� ( Q)���±����У������������ݣ�����������ȷ���ǣ� ��

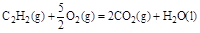 ����H��-1300kJ/mol��˵���У���ȷ���ǣ� ��
����H��-1300kJ/mol��˵���У���ȷ���ǣ� �� ��Ӧ
��Ӧ ��
�� Ϊ
Ϊ