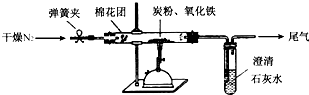
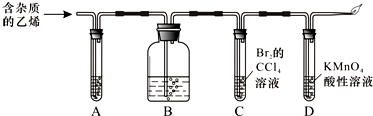
��Ŀ����
14�� �أ�Ga�����ࣨGe�����飨As��������Se����Ϊ�������ڵ�Ԫ�أ������ڸ߿Ƽ���˿�ѧ�ر�����Ϣ�������Ź㷺����;���Իش��������⣺
�أ�Ga�����ࣨGe�����飨As��������Se����Ϊ�������ڵ�Ԫ�أ������ڸ߿Ƽ���˿�ѧ�ر�����Ϣ�������Ź㷺����;���Իش��������⣺��1���ء��ࡢ�顢���ĵ�һ�������ɴ�С��˳����As��Se��Ge��Ga������Ԫ�ط��ű�ʾ��
��2�������ᣨH2SeO3����һ�־綾���ʣ�SeO32-����ԭ�ӵ��ӻ���ʽΪsp3��
��3��AsH3�е��NH3�ͣ���ԭ����NH3���Ӽ����γ��������As�縺��С���뾶���Ӽ䲻���γ������
��4����֪�黯�صľ����ṹ��ͼ�����黯�صĻ�ѧʽΪGaAs��
��5����700��ʱ������CH3��3Ga��AsH3��Ϸ�Ӧ���Ʊ��黯�أ��÷�Ӧ�Ļ�ѧ����ʽΪ��CH3��3Ga+AsH3$\stackrel{700��}{��}$GaAs+3CH4��
���� ��1��ͬ����Ԫ�ش����ҵ�һ������������As��4p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬����һ�����ܽϸߣ�
��2������SeO32- �е�Seԭ�ӵļ۲���Ӷ��������ж��ӻ���ʽ��
��3�����Ƿ��γ�����ĽǶȷ�����
��4�����ݾ����ṹ�����þ�̯������������и�ԭ�Ӹ����ȣ�����ȷ����ѧʽ��
��5������Ԫ���غ��д����ѧ����ʽ��
��� �⣺��1��ͬ����Ԫ�ش����ҵ�һ������������As��4p�ܼ�����3�����ӣ�Ϊ�����ȶ�״̬����һ�����ܽϸߣ����һ������As��Se��Ge��Ga��
�ʴ�Ϊ��As��Se��Ge��Ga��
��2������SeO32- �е�Seԭ�ӵļ۲���Ӷ���Ϊ$\frac{6+2}{2}$=4��������ԭ�ӵ��ӻ���ʽΪ�����ж��ӻ���ʽΪsp3��
�ʴ�Ϊ��sp3��
��3��Nԭ�Ӱ뾶��С���縺�Խϴ�Ӧ��NH3���Ӽ����γ�������е�ϸߣ���As�縺��С���뾶���Ӽ䲻���γ�������е�ϵͣ�
�ʴ�Ϊ��NH3���Ӽ����γ��������As�縺��С���뾶���Ӽ䲻���γ������
��4�����ݾ����ṹ�����þ�̯����֪���������顢��ԭ�Ӹ����ֱ�Ϊ4��$8��\frac{1}{8}+6��\frac{1}{2}$=4�����ǵĸ�����Ϊ1��1�������仯ѧʽΪGaAs��
�ʴ�Ϊ��GaAs��
��5����700��ʱ������CH3��3Ga��AsH3��Ϸ�Ӧ���Ʊ��黯�أ��÷�Ӧ�Ļ�ѧ����ʽΪ��CH3��3Ga+AsH3$\stackrel{700��}{��}$GaAs+3CH4��
�ʴ�Ϊ����CH3��3Ga+AsH3$\stackrel{700��}{��}$GaAs+3CH4��
���� ���⿼���Ϊ�ۺϣ��漰Ԫ�������ɵĵݱ���ɡ��ӻ����͵��жϡ�����������ļ����֪ʶ����Ŀ�Ѷ��еȣ�ע���йػ���֪ʶ�Ļ��ۣ�

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�| A�� | 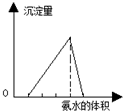 ͼ�ɱ�ʾ���ữ��AlCl3��Һ����μ���ϡ��ˮ���������백ˮ����Ĺ�ϵ | |
| B�� | 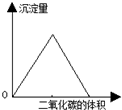 ͼ�пɱ�ʾ�����ʯ��ˮ��ͨ�������̼���壬�������������̼����Ĺ�ϵ | |
| C�� |  ͼ�пɱ�ʾ����������Һ��ͨ�����⣬����������������Ĺ�ϵ | |
| D�� | 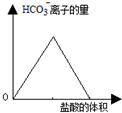 ͼ�пɱ�ʾ��̼������Һ����μ���ϡ���ᣬHCO3-���ӵ�������������Ĺ�ϵ |
| A�� | ����ˮ�⣺S2-+2H2O?H2S+2OH- | |
| B�� | ��Ba��OH��2��Һ�еμ�ϡH2SO4��H++OH-�TH2O | |
| C�� | ��CuCl2��Һ�м���H2S��Һ��Cu2++S2-�TCuS�� | |
| D�� | CaCO3����ϡHCl��CaCO3+2H+�TCa2++CO2��+H2O |
 ����Ȼ����TiO2�н��ʯ�����ѿ����ѿ����־��ͣ����н��ʯ�ľ�����ͼ��ʾ����ÿ�������к��е���ԭ����Ϊ��������
����Ȼ����TiO2�н��ʯ�����ѿ����ѿ����־��ͣ����н��ʯ�ľ�����ͼ��ʾ����ÿ�������к��е���ԭ����Ϊ��������| A�� | 2 | B�� | 4 | C�� | 6 | D�� | 8 |
| A�� | ҽ�þƾ����õ�����ֲ��;������Ƴɣ�Ũ��ͨ����75% | |
| B�� | ��ˮ�м��뾻ˮ����������ʹ��ˮ���� | |
| C�� | �������ֿɽ��Ʊ걾����������ʹ�����ʱ��Ե����� | |
| D�� | Ѥ���ͷ��̻��������˺��ء��ơ��ơ�ͭ�Ƚ���Ԫ�صĻ����� |
| A�� | ԭ�Ӱ뾶��Y��Z��R��T | |
| B�� | XR2��WR2����������R�Ļ��ϼ���ͬ | |
| C�� | ����������Ӧˮ����ļ��ԣ�X��Z | |
| D�� | ��̬�⻯����ȶ��ԣ�W��R��T |
| A�� | ${\;}_{48}^{95}$Cd��${\;}_{48}^{97}$Cd��Ϊͬ�������� | |
| B�� | ${\;}_{48}^{95}$Cd��${\;}_{48}^{97}$Cd��������ͬ | |
| C�� | ${\;}_{48}^{95}$Cd��${\;}_{48}^{97}$Cd��ͬһ�ֺ��� | |
| D�� | ${\;}_{48}^{95}$Cd��${\;}_{48}^{97}$Cd��Ϊͬλ�� |