��Ŀ����
9�����ֻ�����A��Bֻ��C��H��O����Ԫ�أ���A��B���������ʵ����Ȼ�ϣ���ȫȼ�պ�����CO2�������������O2�����2����ͬ��ͬѹ�²ⶨ���������ǵķ�����ɷ���ͨʽ����CO��x��H2O��y��x��yΪ������������֪A��B����Է��������ֱ���a��b����a��b���밴Ҫ��ش��������⣺
��1����A��B��������ԭ������ͬ��A��B��Է��������IJ�ֵһ���ǣ���һ�����֣�28����������
��2����д�����ϴ�������A��B����Է���������С�Ļ�����ķ���ʽ��AC2H2O3��BCH2O2���ṹ��ʽ��AHOOC-CHO��BHCOOH��
��3��д���루2����B��Է����������14���Ҳ�����ͬ�����ʵ����ַ��ӵĽṹ��ʽ��CH3OH��HCOOCH3��
���� ��1��������ɷ���ͨʽ����CO��x��H2O��y��x��yΪ������������A��B��������ԭ������ͬ����y��ͬ����A��B��Է��������IJ�ֵΪ28x��
��2��������ɷ���ͨʽ����CO��x��H2O��y��x��yΪ����������A��B����Է��������ֱ���a��b����a��b����x=y=1ʱB����Է���������С����BΪCH2O2��y=2ʱ���������������ʣ���x=2ʱA����Է���������С����AΪC2H2O3��
��3���루2����B��Է����������14����BΪCH4O��C2H4O2��
��� �⣺��1��������ɷ���ͨʽ����CO��x��H2O��y��x��yΪ������������A��B��������ԭ������ͬ����y��ͬ����A��B��Է��������IJ�ֵΪ28x���ʴ�Ϊ��28��
��2��������ɷ���ͨʽ����CO��x��H2O��y��x��yΪ����������A��B����Է��������ֱ���a��b����a��b����x=y=1ʱB����Է���������С����BΪCH2O2��y=2ʱ���������������ʣ���x=2ʱA����Է���������С����AΪC2H2O3��A�Ľṹ��ʽΪHOOC-CHO��B�Ľṹ��ʽΪ��HCOOH���ʴ�Ϊ��C2H2O3��CH2O2��HOOC-CHO��HCOOH��
��3���루2����B��Է����������14����BΪCH4O��C2H4O2�����Dz�ͬ�����ʣ���ṹ��ʽΪ��CH3OH��HCOOCH3���ʴ�Ϊ��CH3OH��HCOOCH3��
���� ���⿼���л������ʽ��ȷ����������ѧ���ķ������������Ŀ��飬����Ĺؼ�Ϊ�������ͨʽ���з�������Ѷ��еȣ�

 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�| A�� | ��Ca��ClO��2��Һ��ͨ������SO2��Ca2++2ClO-+H2O+SO2=CaSO3��+2HClO | |
| B�� | ������������������ͨ���䰱ˮ�У�SO2+NH3•H2O=HSO3-+NH4+ | |
| C�� | ̼�������Һ����������������Һ��Ӧ��NH4++OH-�TNH3•H2O | |
| D�� | ������������ϡ���FeO+2H+��Fe2++H2O |
 ���������ɱ��Ƿ��ȷ�Ӧ����ӦԼΪ23.4kJ/mol������ʵ��˵����������
���������ɱ��Ƿ��ȷ�Ӧ����ӦԼΪ23.4kJ/mol������ʵ��˵����������| A�� | 1��3-������ϩ���������ȷ�Ӧ | |
| B�� | 1��3-������ϩ��̼�ܱ�Ϊ���������Ǽ��γ�һ���µ�˫�������Ǿ�����һ�������ȶ��ṹ | |
| C�� | 1��3-������ϩ�������ȶ� | |
| D�� | 1��3-������ϩ�������ɻ����������ȷ�Ӧ |
| A�� | 0.6mol�ƾ� | B�� | ��״����4.48L SO2 | ||
| C�� | 0.36g H2O | D�� | ��NA����ԭ�ӵļ��� |
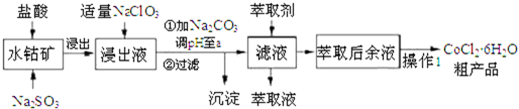
��֪���ٽ���Һ������������Ҫ��H+��Co2+��Fe2+��Mn2+��Al3+�ȣ�
�ڲ��������ӳ���ʱ��Һ��pH���±�������������Ũ��Ϊ��0.01mol/L��
| ������ | Fe��OH��3 | Fe��OH��2 | Co��OH��2 | Al��OH��3 | Mn��OH��2 |
| ��ʼ���� | 2.7 | 7.6 | 7.6 | 4.0 | 7.7 |
| ��ȫ���� | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
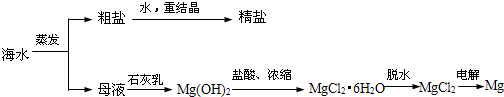
��1��д������������Co2O3������Ӧ�����ӷ���ʽCo2O3+SO32-+4H+=2Co2++SO42-+2H2O��
��2��д��������NaClO3������Ӧ����Ҫ���ӷ���ʽClO3-+6Fe2++6H+=Cl-+6Fe3++3H2O��
��3������Na2CO3��pH��a�����������õ��ij����ɷ�ΪFe��OH��3 ��Al��OH��3��
��4��������1���а���3������ʵ�����������������������Ũ��������ȴ���ᾧ�����ˣ�
��5����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ1������Һ���м�����ȡ����Ŀ���dz�ȥ��Һ�е�Mn2+����ʹ�õ����pH��Χ��B����ѡ����ţ���
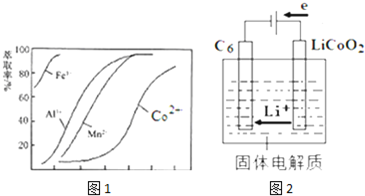
A��2.0��2.5
B��3.0��3.5
C��4.0��4.5
D��5.0��5.5
��6��Ϊ�ⶨ�ֲ�Ʒ��CoCl2•6H2O��������ȡһ�������Ĵֲ�Ʒ����ˮ����������AgNO3��Һ�����ˡ�ϴ�ӣ���������ɺ����������ͨ�����㷢�ֲִ�Ʒ��CoCl2•6H2O��������������100%����ԭ������Ǵֲ�Ʒ���п������Ȼ������ʧȥ�˲��ֽᾧˮ����һ�����ɣ���
��7����֪ij����ӵ��������LiCoO2����Li+�������Ϊ����ʣ����ʱ��Li+��ԭΪLi������ԭ����ʽǶ���ظ�������̼-6��C6���У���ͼ2��ʾ�������õ�ص��ܷ�ӦΪLiCoO2+C6$\frac{\underline{\;���\;}}{�ŵ�}$ CoO2+LiC6������
�ŵ�ʱ��������ӦʽΪCoO2+Li++e-=LiCoO2��
| A�� | ����Ȳ����Ȳ�����ۺϷ�Ӧ�γɵĸ߾��� | |
| B�� | ����Ȳ�Ļ�ѧʽΪ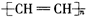 | |
| C�� | ����Ȳ��һ��̼ԭ��֮���Ե�˫�������ϵ���״�ṹ������ | |
| D�� | �������ľ���Ȳ����ϩ�ֱ���ȫȼ��ʱ�����Ǻ�������ͬ |
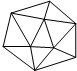 ������Ľṹ��ͼ��ʾ����֪������ṹ��Ԫ������ԭ����ɵ�����ʮ���壬������20���ȱ������ε����һ����Ŀ�Ķ��㣬ÿ������ϸ���1��Bԭ�ӣ������й�˵������ȷ���ǣ�������
������Ľṹ��ͼ��ʾ����֪������ṹ��Ԫ������ԭ����ɵ�����ʮ���壬������20���ȱ������ε����һ����Ŀ�Ķ��㣬ÿ������ϸ���1��Bԭ�ӣ������й�˵������ȷ���ǣ�������| A�� | ÿ������Ӻ���12����ԭ�� | B�� | �������ǿռ���״�ṹ | ||
| C�� | �������м�����60�� | D�� | ÿ������Ӻ���30������ |
| A�� | SO2�����嵥��ʱ������Ӧ�Ļ�ѧ����ʽΪ��2H2O+SO2+Br2�TH2SO3+2HBr | |
| B�� | ��ˮ��ͨ������ʱ������Ӧ�����ӷ���ʽΪ��2Br-+Cl2�TBr2+2Cl- | |
| C�� | Cl-�Ľṹʾ��ͼΪ�� | |
| D�� | ��ˮ��Br-�ĵ���ʽΪ��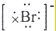 |