��Ŀ����
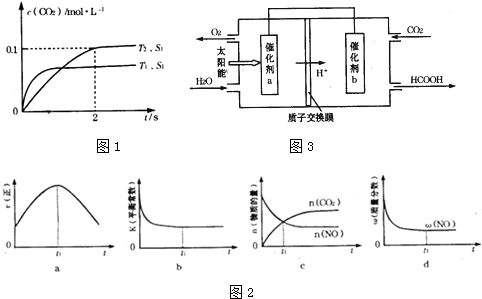
18��2013������������������Ű�ҹ��ж������������У�����β����ȼúβ������ɿ�����Ⱦ��ԭ��֮һ����1������β����������Ҫԭ��Ϊ��2NO��g��+2CO��g��$\stackrel{����}{?}$2CO2��g��+N2��g�������ܱ������з����÷�Ӧʱ��c��CO2�����¶ȣ�T���������ı������S����ʱ�䣨t���ı仯���ߣ���ͼ1��ʾ��
�ݴ��жϣ�
�ٸ÷�Ӧ�ġ�H��0�������������������
����B�¶��£�0��2s�ڵ�ƽ����Ӧ����v��N2��=0.025mol/L��s��
�۵��������������һ��ʱ�����������������ѧ��Ӧ���ʣ��������ı����S1��S2������ͼ�л���c��CO2����T1��S2�����´ﵽƽ������еı仯���ߣ�
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ͼ2��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬����bd ������ţ���
��2��ֱ���ŷ�úȼ�ղ������������������صĻ������⣮
��úȼ�ղ����������������������CH4����ԭNOX�������������������Ⱦ��
���磺CH4��g��+2NO2��g���TN2��g��+CO2��g��+2H2O��g����H1=-867kJ/mol
2NO2��g��?N2O4��g����H2=-56.9kJ/mol
д��CH4��g������ԭN2O4��g������N2��g����H2O��g�����Ȼ�ѧ����ʽCH4��g��+N2O4��g��=N2��g��+CO2��g��+2H2O��g����H=-810.1KJ/mol��
�ڽ�ȼú�����Ķ�����̼�������ã��ɴﵽ��̼�ŷŵ�Ŀ�ģ�ͼ3��ͨ���˹�������ã���CO2��H2OΪԭ���Ʊ�HCOOH��O2��ԭ��ʾ��ͼ������b���淢���ĵ缫��ӦʽΪCO2+2H++2e-=HCOOH��
�۳����£�0.1mol/L��HCOONa��ҺpHΪ10����HCOOH�ĵ��볣��Ka=10-7��
���� ��1���ٸ��ݵ���ƽ���ʱ���ж��¶ȸߵͣ�����ƽ��ʱ������̼��Ũ���ж��¶ȶ�ƽ���Ӱ�죻
����ͼ��֪��T2�¶�ƽ��ʱ��������̼��Ũ�ȱ仯��Ϊ0.1mol/L������v=$\frac{��c}{��t}$����v��CO2�����ٸ�������֮�ȵ��ڻ�ѧ������֮�ȼ���v��N2����
�۽Ӵ����Խ��Ӧ����Խ�죬����ƽ���ʱ��Խ�̣������ı����S1��S2��S2�����´ﵽƽ������ʱ���������������Ӱ��ƽ���ƶ���ƽ��ʱ������̼��Ũ�����¶�T1����ƽ��ʱ��ͬ��
��a������ƽ���������������ȣ����ٱ仯��
b������ƽ����¶�Ϊ��ֵ��ƽ�ⳣ�����䣬��Ϸ�Ӧ���ж��淴Ӧ�����������¶ȱ仯���ж��¶ȶԻ�ѧƽ�ⳣ����Ӱ�죻
c��t1ʱ�̺������̼��NO�����ʵ��������仯������ٱ仯��
d������ƽ������ֵĺ����������仯��
��2���ٸ��ݸ�˹���ɣ�����֪�Ȼ�ѧ����ʽ�����ʵ���ϵ�����мӼ�����Ŀ���Ȼ�ѧ����ʽ��
����ͼ��֪������Ͷ��ˮ�����������������ӣ�����a���淢��������Ӧ��Ϊ����������ͨ�������̼����������������HCOOH��
�ۼ���ˮ��ƽ�ⳣ��Kh���ٸ���Ka=$\frac{K{\;}_{w}}{K{\;}_{h}}$���㣮
��� �⣺��1���ٸ���ͼ��֪�����ȹ���ƽ��ֵ��T1����T2�������¶ȣ�������̼��Ũ�Ƚ��ͣ�ƽ�����淴Ӧ�����ƶ���˵������Ӧ�Ƿ��ȷ�Ӧ������H��0���ʴ�Ϊ������
���ȸ���ͼ����������̼�ķ�Ӧ���ʣ�v��CO2��=$\frac{0.1-0}{2}$=0.05mol/L��s��ͬһ��ѧ��Ӧ��ͬһʱ����ڣ������ʵķ�Ӧ����֮�ȵ��ڼ�����֮�ȣ�����v��N2��=0.025mol/L��s���ʴ�Ϊ��0.025mol/L��s��
�۽Ӵ����Խ��Ӧ����Խ�죬����ƽ���ʱ��Խ�̣������ı����S1��S2��S2�����´ﵽƽ������ʱ���������������Ӱ��ƽ���ƶ���ƽ��ʱ������̼��Ũ�����¶�T1����ƽ��ʱ��ͬ����c��CO2����T1��S2�����´ﵽƽ������еı仯����Ϊ�� ��
��
�ʴ�Ϊ�� ��
��
��a������ƽ���������������ȣ����ٱ仯��t1ʱ��V�����֮���淴Ӧ�������ʷ����仯��δ����ƽ�⣬��a����
b���÷�Ӧ����ӦΪ���ȷ�Ӧ���淴Ӧ�����¶����ߣ���ѧƽ�ⳣ����С������ƽ����¶�Ϊ��ֵ������ߣ�ƽ�ⳣ�����䣬Ϊ��С��ͼ����ʵ�ʷ��ϣ���b��ȷ��
c��t1ʱ�̺������̼��NO�����ʵ��������仯��t1ʱ��δ����ƽ��״̬����c����
d��NO����������Ϊ��ֵ��t1ʱ�̴���ƽ��״̬����d��ȷ��
�ʴ�Ϊ��bd��
��2������֪����CH4��g��+2NO2��g���TN2��g��+CO2��g��+2H2O��g����H1=-867kJ/mol
��2NO2��g��?N2O4��g����H2=-56.9kJ/mol
���ݸ�˹���ɣ���-���CH4��g��+N2O4��g��=N2��g��+CO2��g��+2H2O��g�����ʡ�H=-867kJ/mol-��-56.9kJ/mol��=-810.1kJ/mol��
��CH4��g��+N2O4��g��=N2��g��+CO2��g��+2H2O��g������H=-810.1kJ/mol��
�ʴ�Ϊ��CH4��g��+N2O4��g��=N2��g��+CO2��g��+2H2O��g������H=-810.1kJ/mol��
����ͼ��֪������Ͷ��ˮ�����������������ӣ�����a���淢��������Ӧ��Ϊ����������ͨ�������̼����������������HCOOH���缫��ӦʽΪCO2+2H++2e-=HCOOH��
�ʴ�Ϊ��CO2+2H++2e-=HCOOH��
�۳����£�0.1mol/L��HCOONa��ҺpHΪ10����Һ�д���HCOO-ˮ��HCOO-+H2O?HCOOH+OH-����Kh=$\frac{10{\;}^{-4}��10{\;}^{-4}}{0.1-10{\;}^{-4}}$=10-7����HCOOH�ĵ��볣��Ka=$\frac{K{\;}_{w}}{K{\;}_{h}}$=$\frac{10{\;}^{-14}}{10{\;}^{-7}}$=10-7��
�ʴ�Ϊ��10-7��
���� ���⿼�黯ѧƽ��ͼ��ѧ��Ӧ���ʡ�Ӱ�컯ѧƽ������ء��Ȼ�ѧ����ʽ��д��ԭ��ء�����ƽ�ⳣ����ˮ��ƽ�ⳣ���ȣ���Ŀ�ۺ��Խϴ��Ѷ��еȣ��Ƕ�֪ʶ���ۺ����á�ע�����֪ʶ���������գ�

| A�� | 10ml1.0 mol•L-1��H2SO4��Һ | B�� | 20ml1.0 mol•L-1��Na2CO3��Һ | ||
| C�� | 30ml1.0 mol•L-1��HNO3��Һ | D�� | 40ml1.0 mol•L-1��NaOH��Һ |
| A�� | S����������������ԭ�� | B�� | ����12gCʱ����Ӧת��5mol���� | ||
| C�� | ��ԭ����ֻ��K2S | D�� | KNO3ֻ����������Ӧ |
| A�� | ����Һ��pH=4 | |
| B�� | �ʵ������¶ȣ���Һ��pH���� | |
| C�� | ����ĵ���ƽ�ⳣ��ԼΪ1��10-7 | |
| D�� | ��HX�������c��H+��ԼΪˮ�������c��OH-����106�� |
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | ��������Һ�зֱ���뱥������狀ʹ���Ǧ��Һ | ���й������� | ���������� |
| B | ��ˮ�зֱ���뱽�Ӻͻ���ϩ | ��ˮ����ɫ | �������ӳɷ�Ӧ |
| C | ij��ɫ����ͨ����ˮ�� | ��ˮ��ɫ | ������һ����C2H4 |
| D | ����ˮ��������ϡ�����У� ������Һ���ü�������� | �ж����ЧӦ | �й��ὺ������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
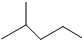 ��A��������2-�����飮 ��A��Ȳ��B�⻯���ɣ���B�Ľṹ��2��
��A��������2-�����飮 ��A��Ȳ��B�⻯���ɣ���B�Ľṹ��2��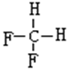 ��
�� F��
F�� ��
�� G��
G�� ��
��

