题目内容
【题目】在容积为1L的密闭容器中,加入5molA物质,在一定条件下同时发生下列两个反应:(1)2A(g)![]() 2B(g)+C(g);(2)A(g)
2B(g)+C(g);(2)A(g)![]() C(g)+D(g)。当达到平衡时,测得c(A)=2.5mol·L-1,c(C)=2.0mol·L-1。则下列说法中正确的是( )
C(g)+D(g)。当达到平衡时,测得c(A)=2.5mol·L-1,c(C)=2.0mol·L-1。则下列说法中正确的是( )
A.达到平衡时c(B)为1.0mol·L-1
B.达到平衡时c(B)=2c(D)
C.达到平衡时c(D)为2.0mol·L-1
D.达到平衡时A的总转化率为40%
【答案】A
【解析】
多反应平衡体系,采用逆推法确定各物质的浓度;容器的体积为1L,设反应(1)中生成的B物质的量为2x,则反应2A(g)![]() 2B(g)+C(g)消耗的n1(A)=2x,生成的n1(C)=x,则反应(2) A(g)
2B(g)+C(g)消耗的n1(A)=2x,生成的n1(C)=x,则反应(2) A(g)![]() C(g)+D(g)消耗的n2(A)=5mol-2.5mol-2x,生成的n2(C)= 5mol-2.5mol-2x,根据题意可知n1(C)+n2(C)=2.0mol,即5mol-2.5mol-2x+x=2mol,解得x=0.5mol;所以平衡时容器内c(A)=2.5mol·L-1,c(C)=2.0mol·L-1,c(B)=1mol·L-1,c(D)=
C(g)+D(g)消耗的n2(A)=5mol-2.5mol-2x,生成的n2(C)= 5mol-2.5mol-2x,根据题意可知n1(C)+n2(C)=2.0mol,即5mol-2.5mol-2x+x=2mol,解得x=0.5mol;所以平衡时容器内c(A)=2.5mol·L-1,c(C)=2.0mol·L-1,c(B)=1mol·L-1,c(D)=![]() =1.5mol·L-1。
=1.5mol·L-1。
A.根据分析可知达到平衡时c(B)为1.0mol·L-1,故A正确;
B.根据分析可知c(B)=1mol·L-1,c(D)=1.5mol·L-1,故B错误;
C.根据分析可知达到平衡时c(D)=1.5mol·L-1,故C错误;
D.A的转化率=![]() ,故D错误。
,故D错误。
故答案为A。

 教学练新同步练习系列答案
教学练新同步练习系列答案 课前课后同步练习系列答案
课前课后同步练习系列答案 课堂小作业系列答案
课堂小作业系列答案【题目】电解质水溶液中存在电离平衡、水解平衡、溶解平衡,请回答下列问题。
(1)已知部分弱酸的电离常数如下表:
弱酸 | HCOOH | HCN | H2CO3 |
电离常数(25 ℃) | Ka=1.77×10-4 | Ka=5.0×10-10 | Ka1=4.3×10-7 Ka2=5.6×10-11 |
①HCOONa、NaCN、NaHCO3、Na2CO3这4种溶液中阴离子结合质子能力最强的是__________。
②体积相同、c(H+)相同的三种酸溶液a.HCOOH;b.HCN;c.H2SO4分别与同浓度的NaOH溶液完全中和,消耗NaOH溶液的体积由大到小的排列顺序是(填字母) ___________。
③向NaCN溶液通入少量CO2反应的化学方程式是_____________________________。
(2)①一定浓度的NaCN溶液pH=9,用离子方程式表示呈碱性的原因是____________;此时c(HCN)/c(CN-)=____________。
②常温下,NaCN与过氧化氢溶液反应,生成NaHCO3和能使湿润的红色石蕊试纸变蓝色的气体,大大降低其毒性。该反应的化学方程式是___________________________。
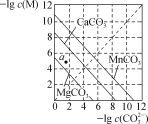
(3)已知CaCO3的Ksp=2.8×10-9,现将浓度为2×10-4 mol·L-1 Na2CO3溶液与CaCl2溶液等体积混合,则生成CaCO3沉淀所需CaCl2溶液的最小浓度为_____________mol·L-1。