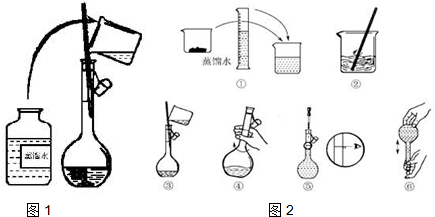
��Ŀ����
4����һƿ�������Һ�����п��ܺ���NH4+��K+��Na+��Ba2+��Al3+��Fe3+��Cl-��I-��NO3-��CO32-��SO42-��MnO4-��ȡ����Һ��������ʵ�飺��1��ȡpH��ֽ���飬��Һ��ǿ���ԣ�
��2��ȡ��������Һ����������CCl4������������ˮ������CCl4����Ϻ�ɫ��
��3����ȡ��������Һ����NaOH��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ������������
��4��ȡ����������������Һ��Na2CO3��Һ���а�ɫ�������ɣ�
��5������3���õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
�ٸ�������ʵ����ʵȷ��������Һ�п϶����ڵ�������NH4+��Ba2+��I-���϶������ڵ�������Al3+��Fe3+��MnO4-��SO42-��CO32-��NO3-��������ȷ���Ƿ���ڵ�������K+��Na+��Cl-
����д����2����4�������з��������ӷ���ʽ��
���裨2��Cl2+2I-�TI2+2Cl-
���裨4��Ba2++CO32-�TBaCO3����
���� ��1����Һ��ǿ���ԣ�˵����Һ�п϶�����H+����H+��CO32-��Ӧ������Ӧ�����ܹ��棻
��2��CCl4����Ϻ�ɫ��˵����I2����������I-�����������������ģ��Ӷ�˵����Һ�к���I-������I-��Ӧ��MnO4-��Fe3+���Ӳ��ܹ��棻
��3������ʵ�飨3��������Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ��������������Fe3+��Al3+����Ӧ����������������ì�ܣ�
��4��ȡ����������������Һ��Na2CO3��Һ���а�ɫ�������ɣ�˵����Һ�п϶�����Ba2+��
��5������3���õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ����������Ϊ�������Դ������
��� �⣺��1����Һ��ǿ���ԣ�˵����Һ�п϶�����H+����H+��CO32-��Ӧ������Ӧ�����ܹ��棬˵����Һ�п϶�������CO32-��
��2��CCl4����Ϻ�ɫ��˵����I2����������I-�����������������ģ��Ӷ�˵����Һ�к���I-����I-��Fe3+��MnO4-��NO3-��H+�ܷ���������ԭ��Ӧ�������ܹ��棬˵����Һ�п϶�������MnO4-��Fe3+��NO3-��
��3������ʵ�飨3��������Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ��������������Fe3+��Al3+����Ӧ����������˵����Һ�п϶�������Fe3+��Mg2+��Al3+��
��4��ȡ����������������Һ��Na2CO3��Һ���а�ɫ�������ɣ�˵����Һ�п϶�����Ba2+����Ba2+����SO42-����������˵����Һ�в���SO42-��
��5������3���õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ��������˵��һ������NH4+��
������������һ�����ڵ�������NH4+��Ba2+��I-��һ�������ڵ�������Al3+��Fe3+��MnO4-��SO42-��CO32-��NO3-������ȷ���Ƿ�K+��Na+��Cl-��
�ʴ�Ϊ��NH4+��Ba2+��I-��Al3+��Fe3+��MnO4-��SO42-��CO32-��NO3-��K+��Na+��Cl-��
�ڣ�2����4�������з��������ӷ���ʽ�ֱ�ΪCl2+2I-�TI2+2Cl-��Ba2++CO32-�TBaCO3�����ʴ�Ϊ��Cl2+2I-�TI2+2Cl-��Ba2++CO32-�TBaCO3����
���� ���⿼�����ʵļ��鼰����Ϊ��Ƶ���㣬���ճ�������֮��ķ�Ӧ�����Ӽ����Ϊ�ƶϵĹؼ������ط������ƶ��������ۺϿ��飬��Ŀ�ѶȲ���

| A�� | ������ѹǿ����ʱ��仯 | |
| B�� | �����ڸ����ʵ�Ũ�Ȳ���ʱ��仯 | |
| C�� | ������X��Y��Z��Ũ��֮��Ϊ1��3��4 | |
| D�� | ��λʱ������0.1 molXͬʱ����0.4molZ |
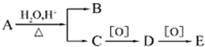 �л���A�ķ���ʽΪC10H20O2���ɷ�����ͼ��ת��������B��E��Ϊͬ���칹����Ϊͬ�����ʣ���A���ܵĽṹ�У�������
�л���A�ķ���ʽΪC10H20O2���ɷ�����ͼ��ת��������B��E��Ϊͬ���칹����Ϊͬ�����ʣ���A���ܵĽṹ�У�������| A�� | 4�� | B�� | 8�� | C�� | 12�� | D�� | 16�� |
| ���� | Na2CO3 | NaHCO3 | NaClO | NaHSO3 |
| pH | 11.6 | 9.7 | 10.3 | 4.0 |
| A�� | 0.1mol/L Na2CO3��Һ��ˮ��ϡ�ͺ���Һ���������ӵ�Ũ�Ⱦ����� | |
| B�� | NaHCO3��Һ�У�c��Na+��+c��H+��=c��HSO3-��+c��OH-��+c��CO32-�� | |
| C�� | NaHSO3��Һ�У�c��Na+����c��HSO${\;}_{3}^{-}$����c��H2SO3����c��SCO${\;}_{3}^{2-}$��+c��OH?�� | |
| D�� | �����£���ͬŨ�ȵ�H2SO3��H2CO3��HClO������Һ��pH��С����H2SO3 |
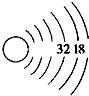 һЩ�����һ����뱣�${\;}_{86}^{222}$Rn�����Ӷ����������Σ������ش�
һЩ�����һ����뱣�${\;}_{86}^{222}$Rn�����Ӷ����������Σ������ش�