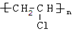��Ŀ����
16��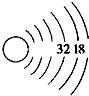 һЩ�����һ����뱣�${\;}_{86}^{222}$Rn�����Ӷ����������Σ������ش�
һЩ�����һ����뱣�${\;}_{86}^{222}$Rn�����Ӷ����������Σ������ش���1����ԭ�ӵ�������Ϊ222��������86��������Ϊ136��
��2������ԭ�ӵĽṹʾ��ͼ��ȫ
��3�������Rn��ԭ�ӽṹԤ������Ļ�ѧ����D
A���dz����ã������������ȷǽ������ʷ�Ӧ
B���Ƚϻ��ã������ƵȽ�����Ӧ
C����̫���ã��뵪����������
D���������������ʷ�����Ӧ
��ѡ���ѡ��������Rn��������Ѵﵽ��8e-���ȶ���
��4���й�${\;}_{86}^{222}$Rn��${\;}_{86}^{220}$Rn��${\;}_{86}^{219}$Rn˵����ȷ����AB
A������ͬ��Ԫ�� B����Ϊͬλ��C������ͬ�ֺ��� D������ͬ��ԭ��
�ɴ˿ɼ�������������Ԫ�����࣬�������������������������࣮
���� ��1��ԭ�ӷ���ZA X��X��ʾԪ�ط��ţ�Z������������A������������A����������=Z����������+N������������
��2��Rn������86�����ӣ����������Ϊ2��8��18��32��18��8��
��3��ϡ������ԭ�ӵ������Ϊ8�����ӣ�����ʧ���ӣ�
��4����������ͬ����������ͬ��ԭ�ӻ���ͬλ�أ���������ͬ��ԭ������ͬ��Ԫ�أ�����������������ͬ����ԭ�����࣮
��� �⣺��1��86222Rn��������Ϊ86��������Ϊ222��������N=A-Z=222-86=136��
�ʴ�Ϊ��222��86��136��
��2��Rn������86�����ӣ����������Ϊ2��8��18��32��18��8������ԭ�ӵĽṹʾ��ͼΪ ��
��
�ʴ�Ϊ�� ��
��
��3��ϡ������ԭ�ӵ������Ϊ8�����ӣ�����ʧ���ӣ��������������ʷ�����Ӧ��
�ʴ�Ϊ��D��Rn��������Ѵﵽ��8e-���ȶ��
��4����������ͬ����������ͬ��ԭ�ӻ���ͬλ�أ���${\;}_{86}^{222}$Rn��${\;}_{86}^{220}$Rn��${\;}_{86}^{219}$Rn����ͬλ�أ���һ��Ԫ�أ���������ͬ��ԭ������ͬ��Ԫ�أ���Ԫ�ص�����������������������������������ͬ����ԭ�����ࣻ
�ʴ�Ϊ��AB��������������������������
���� ���⿼����ԭ�ӽṹ�����ʣ���Ŀ�ѶȲ���ע������ϡ�������ԭ�ӽṹʾ��ͼ�Լ�ͬλ�صĸ��

 ��У����ϵ�д�
��У����ϵ�д�| A�� | Ħ�����������������Ӷ��ٵ�һ�������� | |
| B�� | ��һ�����¶Ⱥ�ѹǿ�£����������Ħ�������� | |
| C�� | �����ӵ���������ֵ��0.012kg14C������ԭ�Ӹ��� | |
| D�� | ������Ħ�����Ϊ22.4L•mol-1������������Ϊ��״�� |
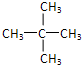
| A�� | C4H8 | B�� | C8H10 | C�� | C2H5Cl | D�� | C2H4Cl2 |
 ��
�� ��
��
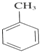 +3HNO3$��_{��}^{Ũ����}$
+3HNO3$��_{��}^{Ũ����}$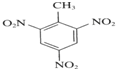 +3H2O
+3H2O