��Ŀ����
��A�����������(Se)����(Te)��Ԫ���ڻ������г����ֳ����ֻ��ϼۣ�����A��Ԫ�صĻ��������о�����������������Ҫ��;����ش��������⣺
��1����Ԫ�����γɷ�����������д��һ����CO2�ȵ��ӵĻ����� ��
��2����Na2O��SiO2��P2O5���������ﰴ�۷е��ɸߵ���˳������ ��
��3��ԭ�ӵĵ�һ��������ָ��̬�����Ի�̬ԭ��ʧȥһ������ת��Ϊ��̬��̬����������Ҫ�����������O��S��Seԭ�ӵĵ�һ�������ɴ�С��˳��Ϊ ��
��4��Seԭ�ӻ�̬������ӵ��Ų�ʽΪ ��H2Se�ķе㣺-41.1�� ��H2S�ķе㣺-60.4�棬�������߷е�������Ҫԭ���� ��
��5��SO32-��������ԭ�ӵ��ӻ���ʽ ,�����ӵ����幹��Ϊ ��
��6��ij����Ԫ��A���������������������������ϡ������������������ľ����ṹ����ͼ��ʾ��
���������Ļ�ѧʽΪ ��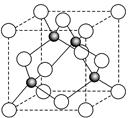
��12�֣�[��4������5������ÿ��1�֣�����ÿ��2��]
(1)CS2��N2O (2)SiO2��Na2O��P2O5 (3) O��S��Se
(4)1s22s22p63s23p63d104s24p4��[Ar]3d104s24p4 H2Se����֮���������ǿ��H2S
(5) sp3�������� (6) AO
���������������1��ԭ�����ͼ۵������ֱ���ȵĻ�Ϊ�ȵ����壬CO2����3��ԭ�ӡ�4��6��2��16���۵�������������CO2�ȵ��ӵĻ�������CS2��N2O��
��2��Na2O��SiO2��P2O5�����������γɵľ������ͷֱ������Ӿ��塢ԭ�Ӿ���ͷ��Ӿ��壬�������������ﰴ�۷е��ɸߵ���˳��������SiO2��Na2O��P2O5��
��3���ǽ�����Խǿ����һ������Խ����Ԫ�������ɿ�֪��ͬ����Ԫ�����϶��·ǽ�������������O��S��Se����Ԫ�صķǽ�����ǿ��˳����O��S��Se����������ԭ�ӵĵ�һ�������ɴ�С��˳��ΪO��S��Se��
��4��SeԪ�ص�ԭ��������34�����Ը��ݹ���ԭ����֪��Seԭ�ӻ�̬������ӵ��Ų�ʽΪ1s22s22p63s23p63d104s24p4��[Ar]3d104s24p4������H2Se��H2S�����γɵľ������;��Ƿ��Ӿ��壬��H2Se����֮���������ǿ��H2S������H2Se�ķ��ӷе����H2S�ķе㡣
��5�����ݼ۲���ӶԻ������ۿ�֪��SO32-��������ԭ�Ӻ��еŶԵ��Ӷ�������6��2��3��2����2��1�����Ը����ӵĿռ乹���������Σ�����ԭ�ӵ��ӻ���ʽ��sp3��
��6�����ݾ����ṹ�������ھ�̯����֪�������к��еİ��������8��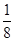 ��6��
��6��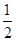 ��4����������ȫ���ھ����ڣ��������4�������Ը�������Ļ�ѧʽ��AO��
��4����������ȫ���ھ����ڣ��������4�������Ը�������Ļ�ѧʽ��AO��
���㣺����ȵ����塢����е�Ƚ��Լ������廯ѧʽȷ������һ�����ܡ���������Ų����ռ乹�͡��ӻ�������͵��жϵ�

 �ִʾ��ƪϵ�д�
�ִʾ��ƪϵ�д�����˵����ȷ����(NAΪ�����ӵ�������ֵ( )
| A��124g P4���е�P-P���ĸ���Ϊ4NA |
| B��12 gʯī�к���C-C���ĸ���Ϊ2NA |
| C��12g���ʯ�к���C-C���ĸ���Ϊ2NA |
| D��60g SiO2�к�Si-O���ĸ���Ϊ2NA |
�����йرȽ��У���С˳�����в���ȷ���ǣ� ��
| A�����ʵ��۵㣺ʯӢ>ʳ��>�� |
| B�����ȶ��ԣ�CH4>H2S>HBr>NH3 |
| C����ɢϵ�з�ɢ�����ӵ�ֱ����Fe��OH��3����Һ>Fe��OH��3����>FeCl3��Һ |
| D�����İ뾶��Cl��>Na+> Mg2+> Al3+ |
�����������ʵ���Ҫ���������ʽṹ����ش��������⡣
��1����ͼ��ʯī�Ľṹ���侧���д��ڵ��������� ������ţ� 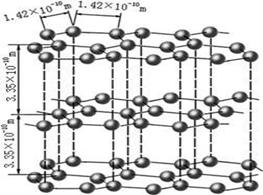
A�Ҽ���B�м���C�����D��λ����E���Ӽ���������F��������G���Ӽ�
��2��������ھ����˵������ȷ����___________
| A�������۵��ɵ͵��ߣ�CF4<CCl4<CBr4<CI4 |
| B��Ӳ���ɴ�С�����ʯ>̼����>����� |
| C���۵��ɸߵ��ͣ�Na>Mg>Al |
| D���������ɴ�С��NaF> NaCl> NaBr>NaI |

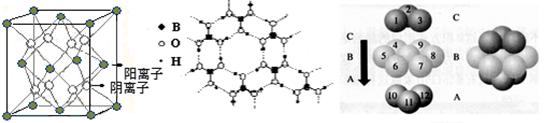
ͼ�� ͼ�� ͼ��
�� ͼI��ʾ�ľ�������Ca2+��������ҵȾ����Ca2+������Ϊ ��
ͼIII��δ��ŵ�Cuԭ���γɾ������Χ����ڵ�Cuԭ����Ϊ ��
��H3BO3������Bԭ���ӻ���ʽ______ ;
�����־������۵�ߵ͵�˳��Ϊ (�ѧʽ)��H3BO3���������ۻ�ʱ���˷�����֮��������Ϊ
��4�� ̼��ij�ֵ��ʵľ�����ͼ��ʾ��һ����������_____��̼ԭ�ӣ����þ�����ܶ�Ϊ�� g/cm3�������ӵ�������ֵΪNA�����������������̼ԭ��֮��ľ���Ϊ_ ____cm(�ô���ʽ��ʾ)
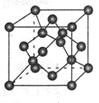
��.�Դ�Ӣ����ѧ�Ұ����У�N.Bartlett���״κϳ��˵�һ��ϡ������Ļ�����XePtF6��������������̷�����믵�һϵ�л������XeF2��XeF4�ȡ�������Ϊ����ϡ�����廯ѧ��������ʷ�Թ��ס�
��1�������XeF4�Ľṹʾ��ͼ��ͼ1���ж���������Ǽ��Է��ӻ��ǷǼ��Է��ӣ�____________________��
��2��XeF2������һ����ɫ���壬ͼ2Ϊ���ľ����ṹͼ��XeF2���������������͵ľ��壿__________________��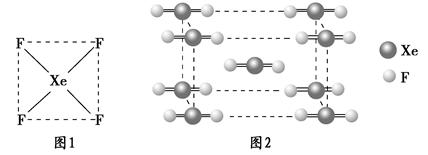
��.��֪�й����ʵ��ۡ��е��������±���
| ���� | MgO | Al2O3 | MgCl2 | AlCl3 |
| �۵�/�� | 2 852 | 2 072 | 714 | 190��2.6��105Pa�� |
| �е�/�� | 3 600 | 2 980 | 1 412 | 182.7 |
��ο�����������պͻش����⣺
��1����ҵ�ϳ��õ������MgCl2�ķ�����������þ���õ��Al2O3�����ʯ���ڻ����ķ�����������Ϊʲô���õ��MgO�ķ�������þ��Ҳ���õ��AlCl3�ķ�����������
___________________________________________________________________________________________________________________________________��
��2����ƿɿ���ʵ��֤��MgCl2��AlCl3�����ľ������ͣ���ʵ�鷽����___________________________________________________________________________________________________________________________________��
ij������A����TK���¾���Ļ����ṹ��Ԫ������ͼ��ʾ��T K����ת��Ϊ����ͼ��ʾ�ṹ�Ļ����ṹ��Ԫ�������־��������ڽ���Aԭ�Ӽ������ͬ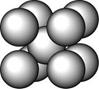

��1����T K���µĴ�A�����У���Aԭ�ӵȾ����������Aԭ����Ϊ______������T K���ϵĴ�A�����У���Aԭ�ӵȾ����������Aԭ����Ϊ___________��
��2����A�����ھ���ת��ǰ�������ṹ��Ԫ�ı߳�֮��Ϊ��TK������TK����֮�ȣ�___________��
��3������ͼ�ĵĶѻ���ʽΪ �� ���ⶨ��ṹ�����ʲ������±���ʾ
| ���� | ���ԭ������ | ���� | �ܶ�/g���M-3 | ԭ�ӻ���/kJ��mol-1 |
| Na | 22.99 | s�� | 0.960 | 108.4 |
| A | 60.20 | d�� | 7.407 | 7735 |
��Aԭ�ӵ�ԭ�Ӱ뾶Ϊ pm���Խ��þ���ԭ�ӻ��Ⱥܸߵ�ԭ�� ��
����֪
 ��7.407��
��7.407�� ,1pm=10
,1pm=10 m��
m�� ���ݱ��е���Ϣ�ж�����˵����ȷ����( )��
| ���� | ���ʯ | ʯī |
| ��� | ��ɫ�������� | �Һڣ��������� |
| �۵� | �� | �� |
| ȼ����/(kJ��mol��1) | 395.4 | 393.5 |
 O2(g)=CO(g) ��H=��393.5kJ��mol-1
O2(g)=CO(g) ��H=��393.5kJ��mol-1B���ɱ�����Ϣ֪C(ʯī,s)=C(���ʯ,s) ��H=��1.9kJ��mol-1
C���ɱ�����Ϣ�ɵ���ͼ��ʾ��ͼ��

D���ɱ�����Ϣ����֪��ͬ�����½��ʯ���۵����ʯī���۵�
��ѧ�д���һЩ�غ��ƽ��ԭ��������������ȷ���� �� ��
| A����������(ԭ��)�غ㶨�ɣ�ij������ȫȼ�յIJ�����CO2��H2O�������һ������ |
| B�����������غ㶨�ɣ����л�ѧ��Ӧ�ķ�Ӧ���������һ������������������� |
| C�����ݵ����غ㶨�ɣ�ԭ����и�����Ӧʧ������һ������������Ӧ�õ����� |
| D�����ݻ�ѧƽ��ԭ�������淴Ӧ������Ӧ�������κ�ʱ��һ�������淴Ӧ���� |
���з�Ӧ������������ԭ��Ӧ���������ȷ�Ӧ����
| A������ϡ����ķ�Ӧ |
| B�����ȵ�ľ̿��CO2��Ӧ |
| C�������������е�ȼ�շ�Ӧ |
| D��Ba(OH)2��8H2O������NH4Cl����ķ�Ӧ |