��Ŀ����
��ʽ̼��ͭ��һ����;�㷺�Ļ���ԭ�ϡ���ҵ�Ͽ������Կ�ʴ��Һ����Ҫ�ɷ���Cu2+��Fe2+��Fe3+��H +��Cl-���Ʊ������Ʊ��������£�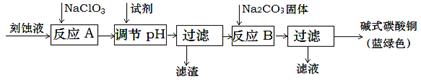
Cu2+��Fe2+��Fe3+���ɳ�����pH���£�
| ���� | Cu(OH)2 | Fe (OH)2 | Fe (OH)3 |
| ��ʼ����pH | 4.2 | 5.8 | 1.2 |
| ��ȫ����pH | 6.7 | 8.3 | 3.2 |
��1�������Ƶ������� ����������Ҫ�ɷ��� ��д��ѧʽ����
��2�����ڷ�ӦA����Һ��pH��ΧӦΪ ������ѡ����Լ��� ������ţ���
a����ˮ b��ϡ���� c���������� d��̼��ͭ
��3����ӦB���¶�����ߣ�����������ɫ��Ʒ�п��ܻ���ֵ������� ����д��ѧʽ��
��4�����˵õ��IJ�Ʒϴ��ʱ������жϲ�Ʒ�Ѿ�ϴ���� ��
��5����Na2CO3��Һ���뵽һ����CuCl2��Һ�еõ�������
�� ������ֻ��CuCO3������Ӧ�����ӷ���ʽΪ ��
�� ������ֻ��Cu(OH)2������Ӧ�����ӷ���ʽ��ʾ����� ��
�� ������Cu(OH)2��CuCO3�Ļ�������ʽ�Σ�����˵�� ��
��6����ʽ̼��ͭ��ɿɱ�ʾΪ��aCuCO3?bCu(OH)2?cH2O����ͨ�����з����ⶨ����ɡ��������£�
�� ������Ʒ���� ���·ֽ⣻�� ���CO2���������� ���ˮ�������������� ����CuO��
��������ⶨ�����������ۡ� ��
��1����Fe2+������Fe3+�����ճ�ȥ��1�֣���Fe(OH)3��1�֣�����2��3.2-4.2��1�֣���d��1�֣�����3��CuO��1�֣�����4��ȡ���һ��ϴ��Һ��������������ϡ���ᣬ���������������ϴ�Ӹɾ�������������Ҳ���֣�1�֣�����5����Cu2++CO32-��CuCO3����1�֣���
��CO32-+H2O OH��+HCO3-��Cu2++2OH����Cu(OH)2����2�֣���
OH��+HCO3-��Cu2++2OH����Cu(OH)2����2�֣���
��̼��ͭ��������ͭ���ܽ���������1�֣���6��ʵ��ֻ��ⶨ�ĸ����е��������ɡ���2�֣�
���������������1�������Ƶ������ǽ�Fe2+������Fe3+�����ճ�ȥ����������Ҫ�ɷ���Fe(OH)3����2�����ڷ�ӦA����Һ��pH��ΧӦΪ3.2-4.2������ѡ����Լ���̼��ͭ���Ҳ������µ����ʡ���3����ӦB���¶�����ߣ���ʽ̼��ͭ���ܻ���ֵ�����������ͭ����4��ȡ���һ��ϴ��Һ��������������ϡ���ᣬ���������������ϴ�Ӹɾ�����5����Na2CO3��Һ���뵽һ����CuCl2��Һ�еõ��������� ������ֻ��CuCO3������Ӧ�����ӷ���ʽΪCu2++CO32-=CuCO3������ ������ֻ��Cu(OH)2������Ӧ�����ӷ���ʽ��ʾ�����CO32-+H2O OH��+HCO3-��Cu2++2OH����Cu(OH)2������ ������Cu(OH)2��CuCO3�Ļ�������ʽ�Σ�����˵��̼��ͭ��������ͭ���ܽ�����������6����ʽ̼��ͭ��ɿɱ�ʾΪ��aCuCO3?bCu(OH)2?cH2O����ͨ�����з����ⶨ����ɡ��������£��� ������Ʒ���� ���·ֽ⣻�� ���CO2���������� ���ˮ�������������� ����CuO��ʵ��ֻ��ⶨ�ĸ����е��������ɡ���
OH��+HCO3-��Cu2++2OH����Cu(OH)2������ ������Cu(OH)2��CuCO3�Ļ�������ʽ�Σ�����˵��̼��ͭ��������ͭ���ܽ�����������6����ʽ̼��ͭ��ɿɱ�ʾΪ��aCuCO3?bCu(OH)2?cH2O����ͨ�����з����ⶨ����ɡ��������£��� ������Ʒ���� ���·ֽ⣻�� ���CO2���������� ���ˮ�������������� ����CuO��ʵ��ֻ��ⶨ�ĸ����е��������ɡ���
���㣺�������ʵĵ��ת��������ؼ��㡣

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���ˮ����þ(MgSO4��7H2O)��ӡȾ����ֽ��ҽҩ�ȹ�ҵ�϶��й㷺��Ӧ�ã����û�����������ɰ�ķ�������þ�����ȡ��ˮ����þ����þ�����Ҫ�ɷ���MgCO3����������������(MgO��SiO2��Fe2O3��FeO��CaO��Al2O3��MnO�ȣ���
��1 ����������������������ʽ��ȫ����ʱ��Һ��pH
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Mn(OH)2 | Mg(OH)2 |
| pHֵ | 5.2 | 3.2 | 9.7 | 10.4 | 11.2 |
��2 �����ε��ܽ��(��λΪg��100gˮ)
| �¶�/�� | 10 | 30 | 40 | 50 | 60 |
| CaSO4 | 0.19 | 0.21 | 0.21 | 0.20 | 0.19 |
| MgSO4��7H2O | 30.9 | 35.5 | 40.8 | 45.6 | / |
��þ����ȡ��ˮ����þ�Ĺ����������£�
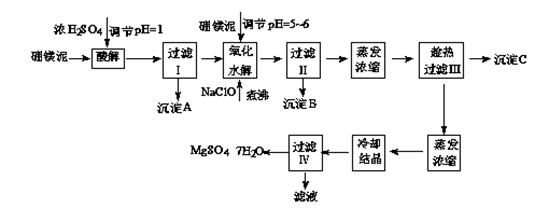
������������ͼ���ο�����pH���ݺ��ܽ�����ݣ��Իش��������⣺
��1������I����Һ�м�����þ�࣬������Һ��pH��5��6���ټ���NaClO��Һ������У�����Һ�е�Mn2+������MnO2����Ӧ�����ӷ�Ӧ����ʽΪ ��������е���ҪĿ���� ��
��2������B�г�MnO2��SiO2����� (�ѧʽ)�����ʡ�
��3��������ˢ�����Һ���Ƿ���Fe3+��ʵ�鷽���� ��
��4������C�Ļ�ѧʽ�� ������III����ȹ��˵�������
����±��ش��������⣺
| �� �� | Fe(OH)2 | Cu(OH)2 | Fe(OH)3 |
| �ܶȻ���25�� | 8.0��10��16 | 2.2��10��20 | 4.0��10��38 |
| ��ȫ����ʱ��pH��Χ | ��9.6 | ��6.4 | 3��4 |
��2���ڿ�����ֱ�Ӽ���CuCl2?2H2O����ò���������ˮCuCl2����CuCl2?2H2O����õ�������ˮCuCl2�ĺ���������___________________________________________��
�����£���a mL ����һԪ��ֱ��NaOH��Һ�������ϣ�ʵ���������£�
| ��� | c(һԪ��) | c(NaOH) /mol/L | �����Һ��pH |
| �� | c(HX)=0��1 mol/L | 0��1 | pH = x |
| �� | c(HY)=0��1mol/L | 0��1 | pH = 7 |
| �� | c(HZ)=0��1 mol/L | 0��1 | pH = 9 |
��1��������������һ������ǿ�����___________��
��2������ʵ�鷢����Ӧ�����ӷ���ʽΪ ��������Һ����ˮ�������c(OH��) = mol/L��
��3��������x��ֵ_____7����>��<��=����
��4������ʵ�鷴Ӧ�����е�pH�仯��������ͼ��
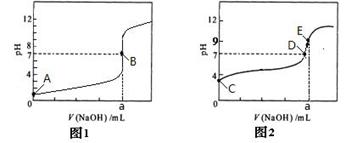
�ٱ�ʾ����ʵ���pH�仯������________________
����ͼ�б�ʾ��Һ�����Եĵ�Ϊ_________����ʾ��Һǡ����ȫ��Ӧ�ĵ���_______
��E���Ӧ����Ũ���ɴ�С��˳��Ϊ ��
��ʳ��Ϊԭ�Ͻ����������ۺ����õ�ijЩ��������ͼ��ʾ��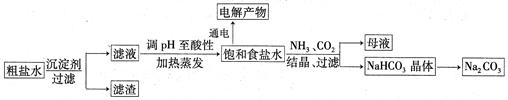
��1��Ϊ��ȥ�����е�Ca2+��Mg2+��SO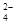 ���õ�������NaCl���壬����������Լ���
���õ�������NaCl���壬����������Լ���
| A��������NaOH��Һ�� | B��������Na2CO3��Һ�� | C����������� | D��������BaCl2��Һ�� |
��2������Һ��pH�������Գ�ȥ��������________��������Ӧ�����ӷ���ʽΪ________��
��3�����ö��Ե缫���200mL1.5mol/Lʳ��ˮ�������2minʱ���������ռ���448mL���壨��״���£���������ǰ����Һ��������䣬��������Һ��pHΪ______��
��4����������NaHCO3������ĸҺ�м��������ʯ�ң���ɻ��һ�ֿ���ѭ��ʹ�õ����ʣ��仯ѧʽ��___________��
��5�������������������й㷺��Ӧ�á�
�ٴ�������ڳ���̨���ۡ���ԭ���ǣ�������ӷ���ʽ������_____________��
�ڳ����£���ijpH=11��Na2CO3��Һ�м������ʯ���飬���˺�������ҺpH=13����Ӧǰ����Һ���뷴Ӧ�����Һ��ˮ�������
 ��OH-���ı�ֵ��_________��
��OH-���ı�ֵ��_________�� ��7�֣����к͵ζ����ⶨ�ռ�Ĵ��ȣ����ռ��к��������Ӧ�����ʣ��Ը���ʵ��ش�
��1����ȷ��ȡ��4.3 g�ռ���Ʒ���Ƴ�250 mL����Һ����Ҫ����Ҫ��������Ͳ���ձ�������������ͷ�ι��⣬�������õ��������� ��
��2��ȡ����Һ10.00 mL���� �ζ�����ȡ����������ƿ�С�
��3������ƿ�еμӼ���ָʾ������0.200 mol��L-1������ζ������ռ���Һ��������ѡ�õ�ָʾ��Ϊ��ѡ����ţ���________����ʯ����Һ ����ɫ��̪ �ۼ�����Һ
��4���������εζ���õ�ʵ���������±����ó��ռ�Ĵ���Ϊ ��
| �ζ����� | ����Һ�����mL�� | �����������mL�� | |
| �ζ�ǰ������mL�� | �ζ��������mL�� | ||
| ��һ�� | 10.00 | 0.50 | 20.40 |
| �ڶ��� | 10.00 | 4.00 | 24.10 |
A ��ʽ�ζ���δ���������Һ��ϴ��ֱ��ע���������Һ
B ��ƿ������ˮϴ����û�и����ע��NaOH����Һ
C װ���������Һ����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D ��ȡHCl��Һ���ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
E�����ռ���ָʾ���ֲ�����ɫ�б仯��ֹͣ�ζ�


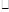 L-1H2SO4ϴ��Fe�ۣ���Ҫ��Ӧ�Ļ�ѧ����ʽΪ ��֮��������ˮϴ�����������ԣ�
L-1H2SO4ϴ��Fe�ۣ���Ҫ��Ӧ�Ļ�ѧ����ʽΪ ��֮��������ˮϴ�����������ԣ�